Brand Name
Lampit
Generic Name
Nifurtimox
View Brand Information FDA approval date: October 01, 2020
Form: Tablet
What is Lampit (Nifurtimox)?
LAMPIT is indicated in pediatric patients (birth to less than 18 years of age and weighing at least.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Phase III Randomized,Multicenter Non-inferiority Study to Evaluate the Efficacy and Safety of Shorter Benznidazole Regimens Compared to the Standard Regimen to Treat Adult Patients With Chronic Chagas Disease
Summary: Chagas disease, a parasitic infection caused by Trypanosoma cruzi, is endemic in much of Latin America and affects people throughout the world. Currently treatment with the only two drugs effective against the infection, benznidazole and nifurtimox, has significant limitations including frequent adverse effects in adult patients. However, timely treatment is key to achieving global objectives of c...
Related Latest Advances
Brand Information
LAMPIT (nifurtimox)
1INDICATIONS AND USAGE
LAMPIT is indicated in pediatric patients (birth to less than 18 years of age and weighing at least 2.5 kg) for the treatment of Chagas disease (American Trypanosomiasis) caused by
2DOSAGE FORMS AND STRENGTHS
LAMPIT tablets are available as 30 mg and 120 mg tablets.
- 30 mg, yellow, round, biconvex tablets, functionally scored on one side for the division of the tablet into equal doses and marked with ‘30’ on the other side.
- 120 mg, yellow, round, biconvex tablets, functionally scored on one side for the division of the tablet into equal doses and marked with ‘120’ on the other side
3CONTRAINDICATIONS
LAMPIT tablets are contraindicated in:
- Patients with known hypersensitivity to nifurtimox or any of the excipients in LAMPIT
- Patients who consume alcohol during treatment
4ADVERSE REACTIONS
The following serious or otherwise important adverse reactions are discussed elsewhere in the labeling:
- Potential for Genotoxicity, Carcinogenicity, and Mutagenicity
- Worsening of Neurological and Psychiatric Conditions
- Hypersensitivity
- Decreased Appetite and Weight Loss
- Porphyria
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect exposure to LAMPIT in one prospective, randomized, double-blind trial (Trial 1). 330 pediatric patients with serologic evidence of
Discontinuation of LAMPIT due to adverse reactions occurred in 14 of 330 (4.2%) patients overall, 12 of 219 (5.5%) patients in the 60-day arm, and 2 of 111 (1.8%) patients in the 30-day arm. Adverse reactions were reported for 213 of 330 (64.5%) patients. The proportion of patients with adverse reactions was higher in the 60-day regimen (67.1%) compared with the 30-day regimen (59.5%). Most patients with adverse reactions had mild (76.5%) or moderate (22.0%) reactions.
The most frequently reported adverse reactions in patients treated with LAMPIT for 60 days were vomiting (14.6%), abdominal pain (13.2%), headache (12.8%), decreased appetite (10.5%), nausea (8.2%), pyrexia (7.3%), rash (5.5%).
Adverse reactions occurring in ≥1% of LAMPIT-treated patients are shown in
Table 3 Adverse Reactions Reported in (≥1%) Pediatric Patients with Chagas Disease in Trial 1 Treated with LAMPIT for 60 days
a Abdominal pain includes abdominal pain and abdominal pain upper
b Rash includes rash, rash macular, rash maculo-papular, rash morbilliform, and rash papular.
Other adverse reactions occurring in 0.1% to less than 1% of patients treated with LAMPIT for 60 days included asthenia, vertigo, arthralgia, myalgia, paresthesia, tremor, irritability, anxiety, pruritus, fatigue, somnolence, seizure, syncope, neutropenia, leukopenia.
4.2Postmarketing Experience
The following safety data were derived during postmarketing surveillance of nifurtimox from outside the United States, including literature data for all age groups (pediatric and adult populations). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Table 4: Postmarketing Adverse Reactions Reported in Pediatric and Adult Populations Treated with Nifurtimox
5DRUG INTERACTIONS
Concomitant use of LAMPIT with alcohol may increase the incidence and severity of undesirable effects similar to other nitrofurans and nitroheterocyclic compounds. LAMPIT is contraindicated in patients who consume alcohol during treatment
6DESCRIPTION
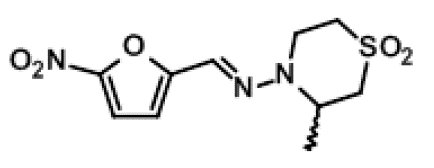
LAMPIT contains nifurtimox, an antiprotozoal. The chemical name is (E)-N-(3-Methyl-1,1-dioxidothiomorpholin-4-yl)-1-(5-nitro-2-furyl)methanimine.
The molecular weight is 287.29 and the molecular formula is C
LAMPIT (nifurtimox) tablets are yellow round, biconvex tablets, each containing 30 mg or 120 mg of nifurtimox, intended for oral use. The 30 mg tablets are functionally scored on one side and marked with ‘30’ on the other side. The 120 mg tablets are functionally scored on one side and marked with ‘120’ on the other side.
The inactive ingredients of the tablets are as follows: calcium hydrogen phosphate dihydrate, magnesium stearate, maize starch, silica colloidal anhydrous and sodium lauryl sulfate.
7CLINICAL STUDIES
Clinical Study Overview
The safety and efficacy of LAMPIT for the treatment of Chagas disease in pediatric patients birth to <18 years of age and weighing at least 2.5 kg were demonstrated in one prospective, randomized, double-blind trial conducted in Argentina, Bolivia and Colombia (Trial 1, NCT02625974). This trial consisted of 2 parts, Part 1 which included treatment with LAMPIT and one year post-treatment follow-up and Part 2, which included an additional three-years of follow up.
Trial 1: Treatment with LAMPIT including One Year Post-treatment Follow-up (Part 1)
Pediatric patients (n=330) with serologic evidence of
Serological response to treatment was defined as ≥20% decrease in optical density measured by lysate and recombinant ELISA in subjects >8 months to <18 years or seroconversion to negative (defined as negative immunoglobulin G concentration in all patients) at 1-year post-treatment follow-up.
The results for both the lysate ELISA and the recombinant ELISA (Table 5) showed superiority in favor of the LAMPIT 60-day arm compared to the LAMPIT 30-day arm (not an approved dosing regimen).
Table 5: Efficacy Results using Lysate ELISA and Recombinant ELISA at 1 year Post-Treatment
The F29 ELISA detects antibodies to recombinant antigens obtained from the flagellar protein F29 of
Trial 1: Additional Three-years of Follow-up (Part 2)
To determine the seroconversion rate in patients treated with LAMPIT at the 4-year time point, a total of 295 (197 in the 60-day regimen and 98 in the 30-day regimen) of the 330 pediatric patients were followed for another 3 years after the end of Part 1. Seroconversion to negative confirmed by three assays, lysate ELISA, recombinant ELISA and IHA, was evaluated in patients in the 60-day treatment arm by age group (Table 6) in Part 2. Patients were considered as seroconverted to negative if all three test results were negative. All seroconversions at 4 years post-treatment were from patients who were <2 years of age at baseline. Nine of 11 (81.8%) patients in the 60-day treatment arm who were <8 months of age at baseline seroconverted to negative at 4 years post-treatment. Among 39 patients in the 60-day treatment arm who were 0 to 4 years of age at baseline, 9 (23.1%) seroconverted for 2 or more consecutive years, which was higher than the 0% conversion rate from historical data
Table 6: Efficacy Results using Seroconversion to Negative Confirmed by Lysate ELISA, Recombinant ELISA and IHA at 4 years Post-Treatment
* A positive
The efficacy of LAMPIT in pediatric patients 5 to <18 years of age was extrapolated from efficacy established in the younger pediatric population between 0 and 4 years of age. Biologically, it is expected that LAMPIT would have the same effect on
8REFERENCES
1. Sosa Estani, S., E. L. Segura, A. M. Ruiz, E. Velazquez, B. M. Porcel and C. Yampotis (1998) Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am J Trop Med Hyg 59(4): 526-529.
2. Suasnábar S, Olivera LV, Arias E, Bizai ML, Bottasso O, Arias E, Fabbro D (2021) Trypanocidal therapy among children infected by
3. Streiger ML, del Barco ML, Fabbro DL, Arias ED, Amicone NA (2004) Longitudinal study and specific chemotherapy in children with chronic Chagas' disease, residing in a low endemicity area of Argentina. Rev Soc Bras Med Trop 37(5):365-375.
9PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Embryo Fetal Toxicity
- Advise pregnant women and females of reproductive potential of the potential risk of LAMPIT to a fetus and to inform their healthcare provider of a known or suspected pregnancy
- Advise females of reproductive potential to use effective contraception while taking LAMPIT and for 6 months after the last dose
- Advise male patients with female partners of reproductive potential to use condoms during treatment with LAMPIT and for 3 months after the final dose of LAMPIT
Lactation
Advise nursing mothers to monitor infants exposed to LAMPIT through breast milk for vomiting, rash, decreased appetite, fever, and irritability
Infertility
Advise males of reproductive potential that LAMPIT may impair fertility
Worsening of Neurological and Psychiatric Conditions
Advise patients with a history of brain injury, seizures, psychiatric disease, serious behavioral alterations or if neurological and/or psychiatric drug reactions occur, that LAMPIT tablets should be administered only under close medical supervision
Hypersensitivity
Inform patients that hypersensitivity could be a reaction caused by LAMPIT or an immune response triggered by Chagas disease during treatment. Hypersensitivity reactions could include hypotension, angioedema (including laryngeal or facial edema), dyspnea, pruritus, rash or other severe skin reactions
Alcohol Consumption
Advise patients to discontinue alcohol use during treatment with LAMPIT. LAMPIT is contraindicated in patients who consume alcohol during treatment
Decreased Appetite and Weight Loss
Inform patients that LAMPIT can cause decreased appetite and weight loss. Body weight should be checked every 14 days, as the dosage may have to be adjusted
Porphyria
Advise patients with porphyria that treatment with nitrofuran derivatives, such as LAMPIT, may precipitate acute attacks of porphyria. Administer LAMPIT tablets under close medical supervision in patients with porphyria.
Important Administration Instructions
Advise patients to take LAMPIT with food.
Advise patients not to break LAMPIT tablets mechanically with a tablet splitting device
For patients who cannot swallow tablets, LAMPIT can be dispersed in water and administered as a slurry, taken with food
Ability to Operate Vehicles or Machinery
Inform patients that if muscle weakness or tremors occur during treatment with LAMPIT they should not drive, cycle or use any tools or machines
Manufactured for:
Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981 USA
© 2023 Bayer HealthCare Pharmaceuticals Inc.
- For more information, call Bayer HealthCare Pharmaceuticals Inc. at Bayer at 1-888-842-2937 or go to
10Patient Package Insert
This Patient Information has been approved by the U.S. Food and Drug Administration Issued:5/2023
11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 50419-750-01
Rx only
Lampit
(nifurtimox) tablets
30 mg
Oral use
100 tablets

12PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 50419-751-01
Rx only
Lampit
(nifurtimox) tablets
120 mg
Oral use
100 tablets

