Brand Name
Corvert
Generic Name
Ibutilide
View Brand Information FDA approval date: December 28, 1995
Classification: Antiarrhythmic
Form: Injection
What is Corvert (Ibutilide)?
CORVERT Injection is indicated for the rapid conversion of atrial fibrillation or atrial flutter of recent onset to sinus rhythm. Patients with atrial arrhythmias of longer duration are less likely to respond to CORVERT. The effectiveness of ibutilide has not been determined in patients with arrhythmias of more than 90 days in duration.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Corvert (ibutilide fumarate)
1DESCRIPTION
CORVERT Injection (ibutilide fumarate injection) is an antiarrhythmic drug with predominantly class III (cardiac action potential prolongation) properties according to the Vaughan Williams Classification. Each milliliter of CORVERT Injection contains 0.1 mg of ibutilide fumarate (equivalent to 0.087 mg ibutilide free base), 0.189 mg sodium acetate trihydrate, 8.90 mg sodium chloride, hydrochloric acid to adjust pH to approximately 4.6, and Water for Injection.
CORVERT Injection is an isotonic, clear, colorless, sterile aqueous solution.
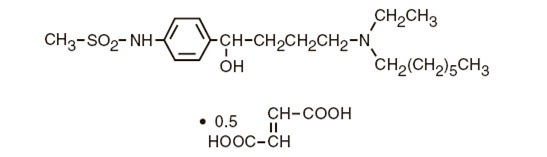
Ibutilide fumarate has one chiral center, and exists as a racemate of the (+) and (−) enantiomers.
The chemical name for ibutilide fumarate is Methanesulfonamide, N-{4-{4-(ethylheptylamino)-1-hydroxybutyl}phenyl}, (+) (−), (E)-2-butenedioate (1:0.5) (hemifumarate salt). Its molecular formula is C
Ibutilide fumarate is a white to off-white powder with an aqueous solubility of over 100 mg/mL at pH 7 or lower.
The structural formula of ibutilide fumarate is represented below:
2INDICATIONS AND USAGE
CORVERT Injection is indicated for the rapid conversion of atrial fibrillation or atrial flutter of recent onset to sinus rhythm. Patients with atrial arrhythmias of longer duration are less likely to respond to CORVERT. The effectiveness of ibutilide has not been determined in patients with arrhythmias of more than 90 days in duration.
3CONTRAINDICATIONS
CORVERT Injection is contraindicated in patients who have previously demonstrated hypersensitivity to ibutilide fumarate or any of the other product components.
4ADVERSE REACTIONS
CORVERT Injection was generally well tolerated in clinical trials. Of the 586 patients with atrial fibrillation or atrial flutter who received CORVERT in phase II/III studies, 149 (25%) reported medical events related to the cardiovascular system, including sustained polymorphic ventricular tachycardia (1.7%) and nonsustained polymorphic ventricular tachycardia (2.7%).
Other clinically important adverse events with an uncertain relationship to CORVERT include the following (0.2% represents one patient): sustained monomorphic ventricular tachycardia (0.2%), nonsustained monomorphic ventricular tachycardia (4.9%), AV block (1.5%), bundle branch block (1.9%), ventricular extrasystoles (5.1%), supraventricular extrasystoles (0.9%), hypotension/postural hypotension (2.0%), bradycardia/sinus bradycardia (1.2%), nodal arrhythmia (0.7%), congestive heart failure (0.5%), tachycardia/sinus tachycardia/supraventricular tachycardia (2.7%), idioventricular rhythm (0.2%), syncope (0.3%), and renal failure (0.3%). The incidence of these events, except for syncope, was greater in the group treated with CORVERT than in the placebo group.
Another adverse reaction that may be associated with the administration of CORVERT was nausea, which occurred with a frequency greater than 1% more in ibutilide-treated patients than those treated with placebo.
The medical events reported for more than 1% of the placebo- and ibutilide-treated patients are shown in the following Table.
In the post-cardiac surgery study (see
5DOSAGE AND ADMINISTRATION
The recommended dose based on controlled trials (see
In a trial comparing ibutilide and sotalol (see
In the post-cardiac surgery study (see
Patients should be observed with continuous ECG monitoring for at least 4 hours following infusion or until QTc has returned to baseline. Longer monitoring is required if any arrhythmic activity is noted. Skilled personnel and proper equipment (see
5.1Dilution:
CORVERT Injection may be administered undiluted or diluted in 50 mL of diluent. CORVERT may be added to 0.9% Sodium Chloride Injection or 5% Dextrose Injection before infusion. The contents of one 10 mL vial (0.1 mg/mL) may be added to a 50 mL infusion bag to form an admixture of approximately 0.017 mg/mL ibutilide fumarate. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
5.2Compatibility and Stability:
The following diluents are compatible with CORVERT Injection (0.1 mg/mL):
- 5% Dextrose Injection
- 0.9% Sodium Chloride Injection
The following intravenous solution containers are compatible with admixtures of CORVERT Injection (0.1 mg/mL):
- polyvinyl chloride plastic bags
- polyolefin bags
Admixtures of the product, with approved diluents, are chemically and physically stable for 24 hours at room temperature (15° to 30° C or 59° to 86° F) and for 48 hours at refrigerated temperatures (2° to 8°C or 36° to 46°F). Strict adherence to the use of aseptic technique during the preparation of the admixture is recommended in order to maintain sterility.
6HOW SUPPLIED
CORVERT Injection (ibutilide fumarate injection) is supplied as an acetate-buffered isotonic solution at a concentration of 0.1 mg/mL that has been adjusted to approximately pH 4.6 in 10 mL clear glass, single-dose, flip-top vials.
Single-dose 10 mL vial, 1 mg /10 mL (0.1 mg/mL) NDC 0009-3794-22
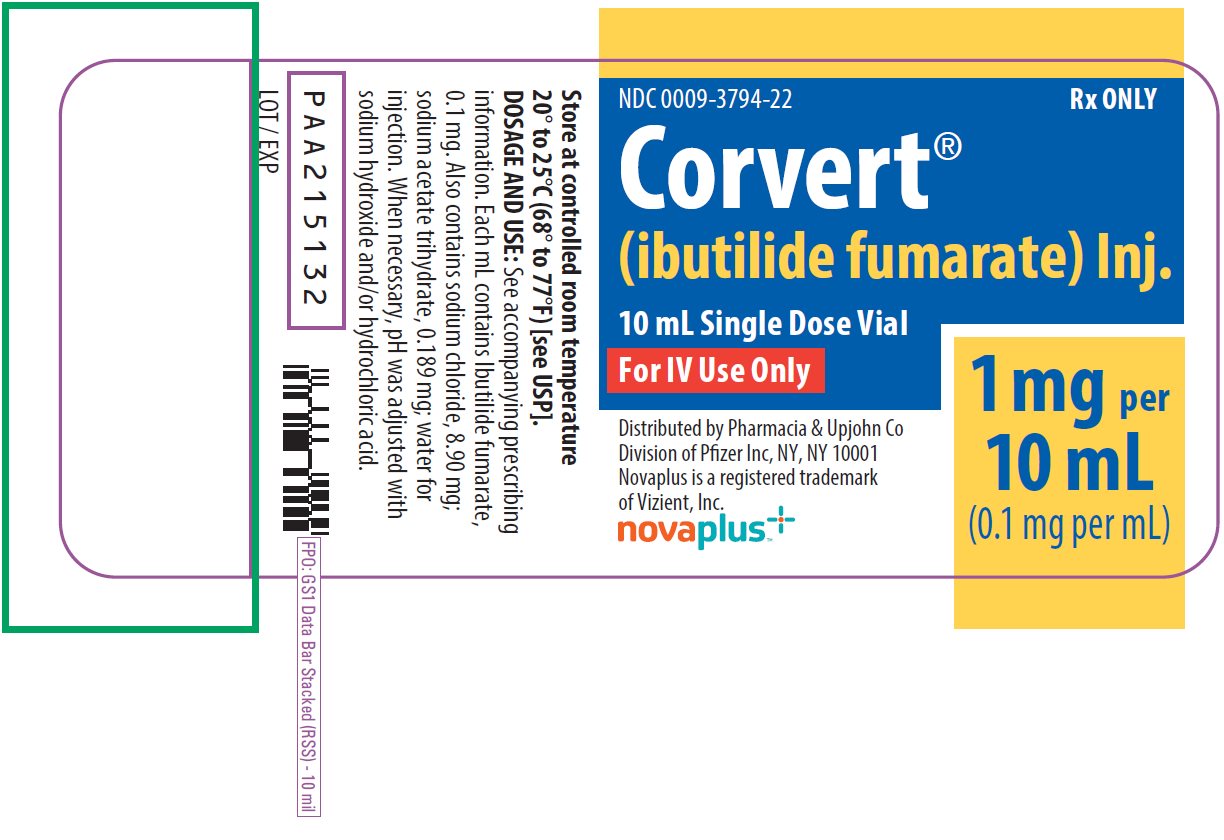
7PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
NDC 0009-3794-22
Corvert
10 mL Single Dose Vial
For IV Use Only
1 mg per
Distributed by Pharmacia & Upjohn Co
novaplus
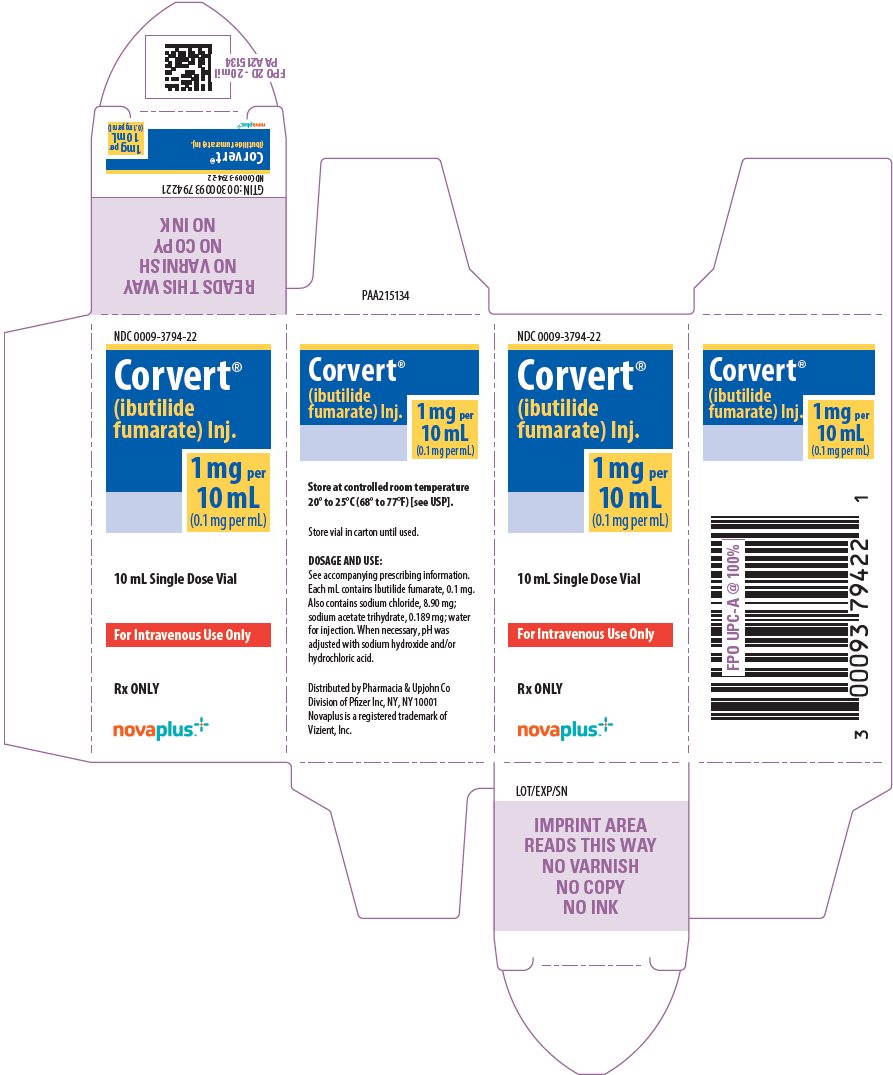
8PRINCIPAL DISPLAY PANEL - 10 mL Vial Carton
NDC 0009-3794-22
Corvert
1 mg per
10 mL Single Dose Vial
For Intravenous Use Only
Rx ONLY
novaplus