Voxzogo
What is Voxzogo (Vosoritide)?
Approved To Treat
Related Clinical Trials
Summary: The purpose of this study is to evaluate the safety and efficacy of daily administration of vosoritide in participants with HCH aged 0 to \< 36 months over a 52-week period.
Summary: The purpose of this basket study in children with Turner syndrome, SHOX deficiency, and Noonan syndrome is to evaluate the effect of 3 doses of vosoritide versus hGH on growth as measured by AGV after 6 months of treatment. The long-term efficacy and safety of vosoritide at the therapeutic dose will be evaluated up to FAH.
Summary: This is an observational study of individuals with achondroplasia in the United States. The primary study population consists of pediatric individuals treated and untreated with VOXZOGO™. Study enrollment started in February 2023. The projected total duration of the study is approximately 5 years at minimum from start of study recruitment in February 2023, with the duration of individual prospecti...
Related Latest Advances
Brand Information
- Risk of Low Blood Pressure
- Wash your hands with soap and water.
- Do not drop VOXZOGO or put opened items down on surfaces that are not clean.
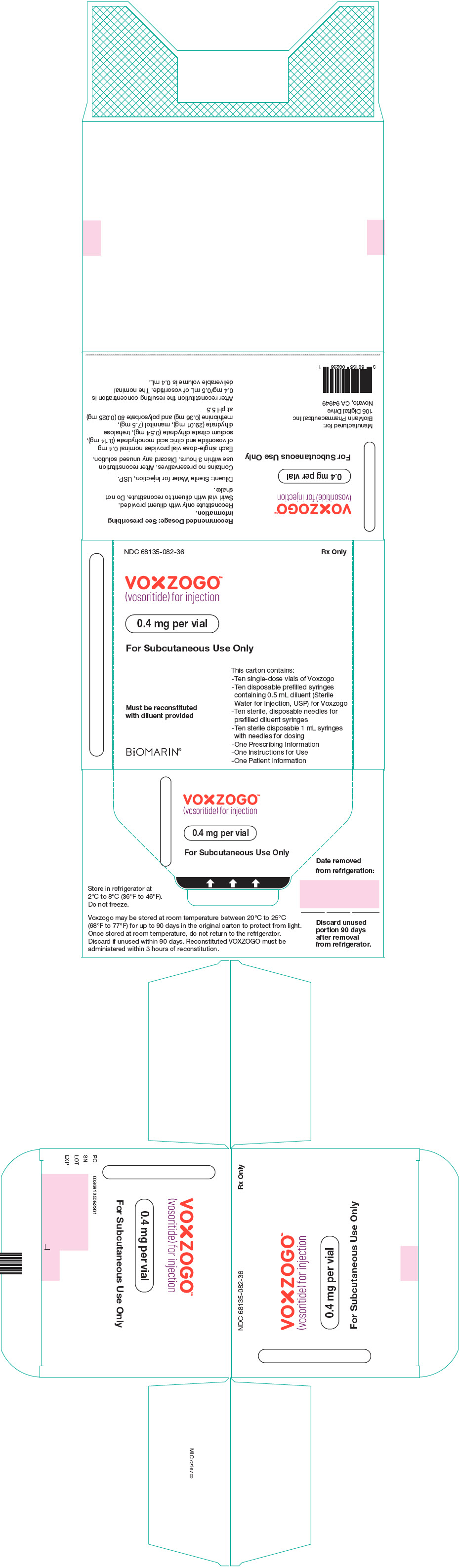
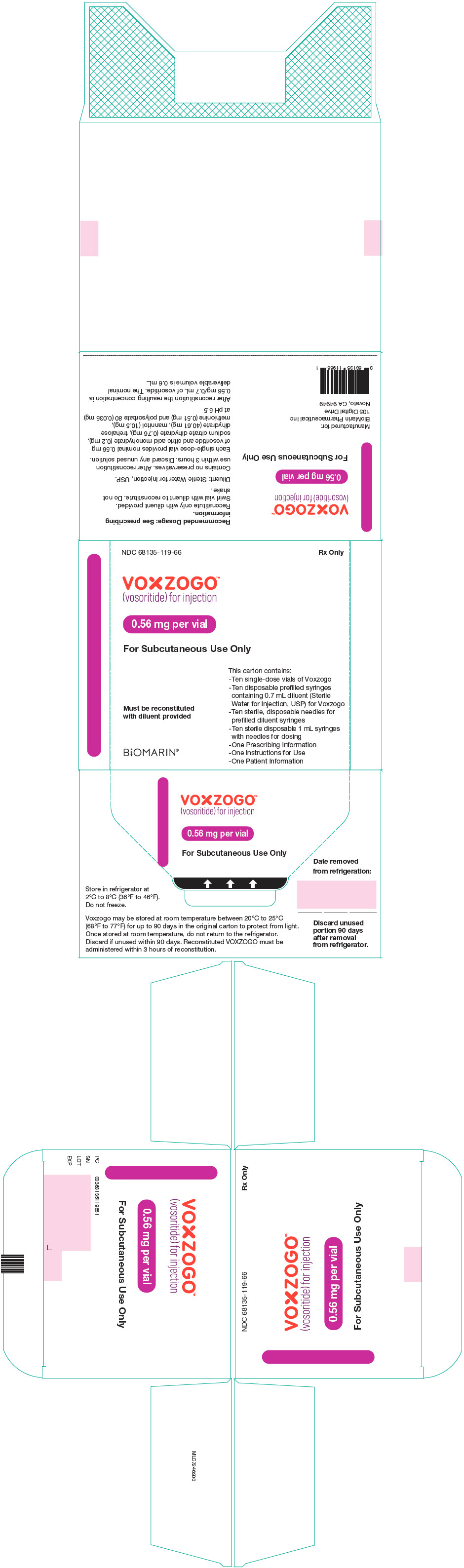
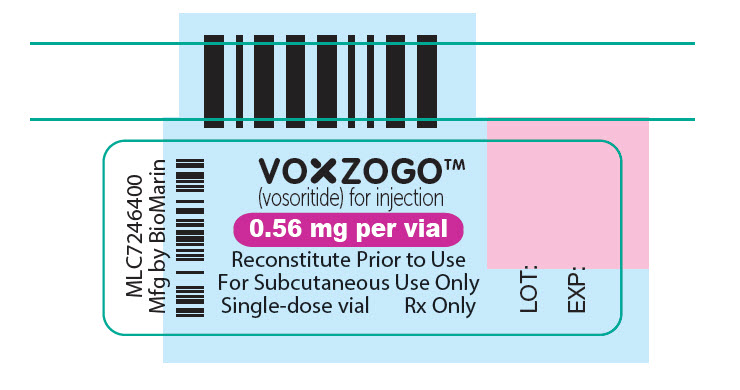
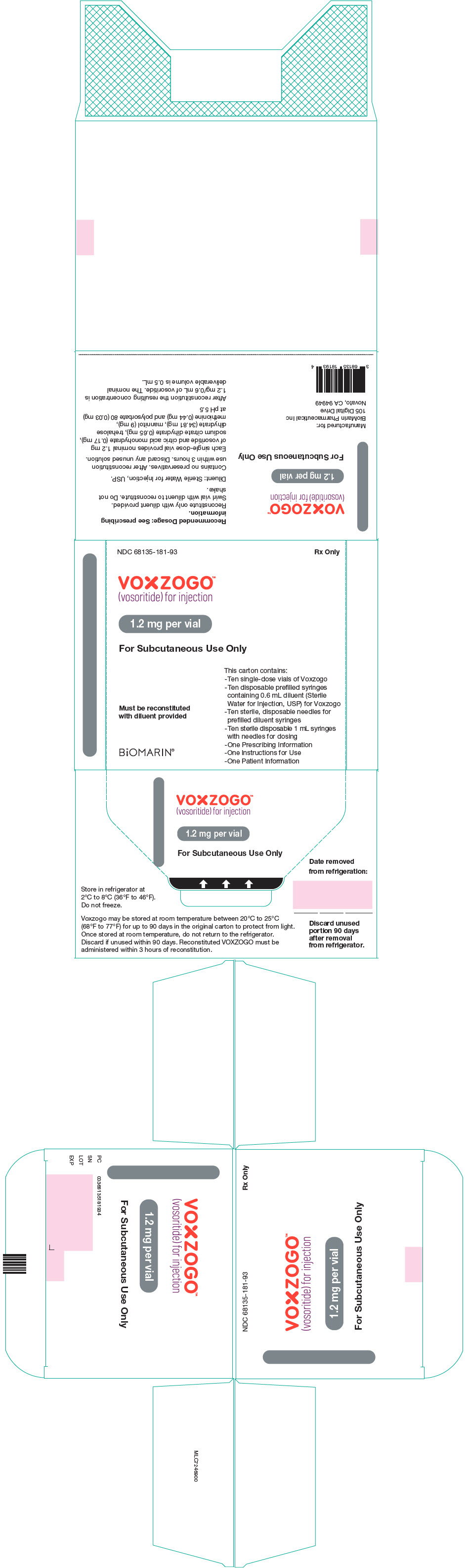
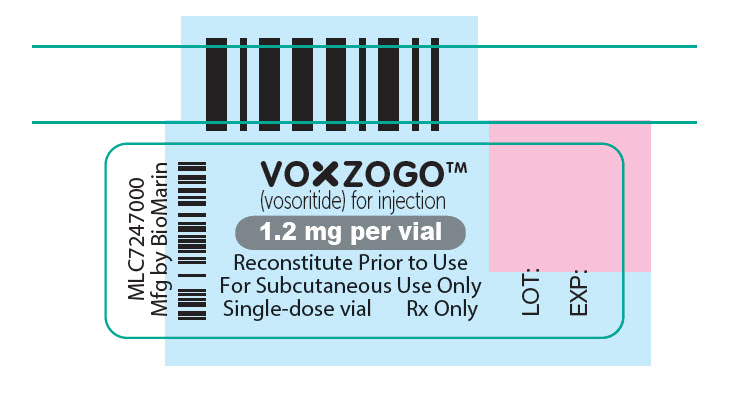
- VOXZOGO is available in more than 1 strength.
- Take the VOXZOGO vial and prefilled diluent syringe out of the refrigerator and allow them to reach room temperature before mixing.
- Inspect the vial and supplies for any signs of damage or contamination. Do not use if damaged or contaminated.
- Check the expiration date. The expiration date can be found on the carton, vial and prefilled diluent syringe. Do not use if expired.
- Your child should eat a meal and drink a glass (about 8 to 10 ounces) of fluid (such as water, milk, or juice) within 1 hour before injection.
- VOXZOGO should be given at about the same time every day.
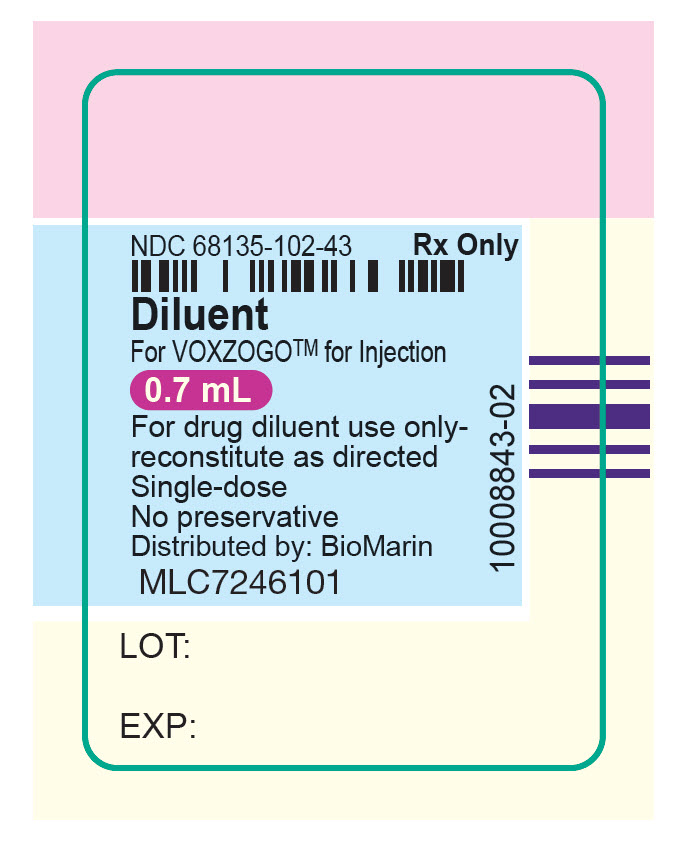
- Do not mix VOXZOGO with other medicines.
- After mixing the VOXZOGO, use it right away. Do not use the mixed VOXZOGO if it has been sitting at room temperature for more than 3 hours. Throw it away (dispose of) in a sharps container. See step 18 and " for more information.
- Do not reuse any of the supplies. After the injection, throw away (dispose of) the used vial even if there is VOXZOGO remaining. See step 18 and " for more information.
- Store the VOXZOGO vial and prefilled diluent syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store VOXZOGO (before mixing) at room temperature between 68°F to 77°F (20°C to 25°C) for 90 days. Record the date you started storing VOXZOGO at room temperature on the carton to keep track of the 90 days.
- Do not freeze VOXZOGO.
- Store VOXZOGO out of direct sunlight.
- is made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid without sharps being able to come out,
- is upright and stable during use,
- is leak-resistant, and
- is properly labeled to warn of hazardous waste inside the container.
- Inspect the injection site. If a small amount of bleeding occurs from the injection site, gently press a gauze pad on it for a few seconds or apply a bandage.
- Monitor for signs of low blood pressure, such as dizziness, tiredness, and nausea. If your child experiences these symptoms you should call your child's healthcare provider, then get your child to lay back with legs raised.
- Call your healthcare provider
- Call BioMarin at 1-800-123-4567
- Visit www.VOXZOGO.com