Brand Name
Daybue
Generic Name
Trofinetide
View Brand Information FDA approval date: March 10, 2023
Form: Solution
What is Daybue (Trofinetide)?
DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older. DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Cognitive Function in Rett Syndrome During Trofinetide Treatment
Summary: Assessing cognitive functions among individuals with severe intellectual and developmental disabilities (IDD), including RTT, is often challenging due to floor effects of many standardized assessment batteries in this population. In addition, deficits in motor function and verbal ability may obscure certain abilities in this population when using standard IQ measures. Remote eye-tracking tasks hav...
Related Latest Advances
Brand Information
Daybue (trofinetide)
1INDICATIONS AND USAGE
DAYBUE and DAYBUE STIX are indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
2DOSAGE FORMS AND STRENGTHS
Oral solution: 200 mg/mL of a pink to red, strawberry flavored solution.
For oral solution: 5,000 mg, 6,000 mg, or 8,000 mg of white, off-white to pinkish powder with strawberry flavor, packaged in individual packets.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Diarrhea
- Weight Loss
- Vomiting
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled and uncontrolled trials in patients with Rett syndrome, 260 patients ages 2 to 40 years were treated with DAYBUE, including 109 patients treated for more than 6 months, 69 patients treated for more than 1 year, and 4 patients treated for more than 2 years.
The safety of DAYBUE STIX has been established from an adequate, well-controlled study, and open-label studies of DAYBUE
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of DAYBUE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Aspiration and aspiration pneumonia secondary to vomiting
5DESCRIPTION
DAYBUE oral solution and DAYBUE STIX for oral solution contain the active moiety trofinetide. The chemical name of trofinetide is (2S)-2-{[(2S)-1-(2-aminoacetyl)-2-methylpyrrolidine-2-carbonyl]amino}pentanedioic acid (IUPAC). The molecular formula of trofinetide is C
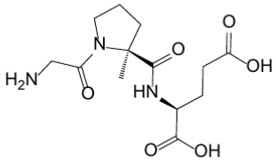
Trofinetide is a white to off-white solid and is freely soluble in water.
DAYBUE oral solution is pink to red in color and contains 1 g of trofinetide in each 5 mL of solution (200 mg/mL). The oral solution also contains FD&C Red No. 40, maltitol, methylparaben sodium, propylparaben sodium, purified water, strawberry flavor, and sucralose as inactive ingredients.
DAYBUE STIX for oral solution is a white, off-white to pinkish powder to be dissolved in a cold to room temperature water or water-based beverage before administration and contains 5,000 mg, 6,000 mg, or 8,000 mg of trofinetide in each packet. The for oral solution powder contains natural strawberry flavor and sucralose as inactive ingredients.
6PATIENT COUNSELING INFORMATION
Advise the caregiver or patient to read the FDA-approved patient labeling (Patient Information).
7PRINCIPAL DISPLAY PANEL – 450 ML ORAL SOLUTION BOTTLE LABEL
NDC 63090-660-01
450 mL
Daybue™
200 mg/mL
Recommended Dosage:
For oral or G-tube administration only.

8PRINCIPAL DISPLAY PANEL – 450 ML ORAL SOLUTION BOTTLE CARTON
NDC 63090-660-01
Daybue™
200 mg/mL
Recommended Dosage:
For oral or G-tube administration only.
450 mL
Rx only

9PRINCIPAL DISPLAY PANEL – 5,000 MG FOR ORAL SOLUTION POWDER PACKET
NDC 63090-663-01
Daybue
5,000 mg
For Oral or G-tube administration only
Keep out of reach of children
Rx Only

10PRINCIPAL DISPLAY PANEL – 5,000 MG FOR ORAL SOLUTION POWDER CARTON
NDC 63090-663-60
Daybue
5,000 mg
For Oral or G-tube
60 Packets
Rx Only

11PRINCIPAL DISPLAY PANEL – 6,000 MG FOR ORAL SOLUTION POWDER PACKET
NDC 63090-664-01
Daybue
6,000 mg
For Oral or G-tube administration only
Keep out of reach of children
Rx Only

12PRINCIPAL DISPLAY PANEL – 6,000 MG FOR ORAL SOLUTION POWDER CARTON
NDC 63090-664-60
Daybue
6,000 mg
For Oral or G-tube
60 Packets
Rx Only

13PRINCIPAL DISPLAY PANEL – 8,000 MG FOR ORAL SOLUTION POWDER PACKET
NDC 63090-665-01
Daybue
8,000 mg
For Oral or G-tube administration only
Keep out of reach of children
Rx Only

14PRINCIPAL DISPLAY PANEL – 8,000 MG FOR ORAL SOLUTION POWDER CARTON
NDC 63090-665-60
Daybue
8,000 mg
For Oral or G-tube
60 Packets
Rx Only
