Brand Name
Qalsody
Generic Name
Tofersen
View Brand Information FDA approval date: April 25, 2023
Classification: Antisense Oligonucleotide
Form: Injection
What is Qalsody (Tofersen)?
QALSODY is indicated for the treatment of amyotrophic lateral sclerosis in adults who have a mutation in the superoxide dismutase 1 gene. This indication is approved under accelerated approval based on reduction in plasma neurofilament light chain observed in patients treated with QALSODY. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trial. QALSODY is an antisense oligonucleotide indicated for the treatment of amyotrophic lateral sclerosis in adults who have a mutation in the superoxide dismutase 1 gene. This indication is approved under accelerated approval based on reduction in plasma neurofilament light chain observed in patients treated with QALSODY. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trial.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
QALSODY (tofersen)
1INDICATIONS AND USAGE
QALSODY is indicated for the treatment of amyotrophic lateral sclerosis (ALS) in adults who have a mutation in the superoxide dismutase 1 (
2DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/15 mL (6.7 mg/mL) as a clear and colorless to slightly yellow solution in a single-dose vial.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
- Myelitis and/or Radiculitis
- Papilledema and Elevated Intracranial Pressure
- Aseptic Meningitis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of QALSODY cannot be directly compared to rates in clinical trials of other drugs and may not reflect the rates observed in practice.
The safety of QALSODY 100 mg was evaluated in 147 patients with SOD1-ALS. The median patient exposure was 119.4 weeks (range from 4 to 212 weeks). QALSODY was evaluated in the placebo-controlled Study 1 and in the open label extension Study 2. In Study 1 Part C, approximately 43% were female; 57% were male; 64% were White and 8% were Asian. The mean age at entry in Study 1 Part C was 49.8 years (range from 23 to 78 years).
The most common adverse reactions (≥ 10% of patients treated with QALSODY and greater than placebo) were pain, fatigue, arthralgia, CSF white blood cell increased, and myalgia.
5DESCRIPTION
Tofersen, an antisense oligonucleotide, is a 20-base residue (20-mer) 5-10-5 MOE gapmer mixed backbone oligonucleotide. Of the nineteen internucleotide linkages, fifteen are 3′-O to 5′-O phosphorothioate diesters, and four are 3′-O to 5′-O phosphate diesters. Ten of the twenty sugar residues are 2-deoxy-D-ribose and the remainder are 2′-O-(2-methoxyethyl)-D-ribose (MOE). The residues are arranged so that there are five MOE nucleosides at the 5′ and 3′-ends of the molecule flanking a gap of ten 2′-deoxynucleosides. The cytosine and uridine bases are methylated at the 5-position. The structural formula is:
Figure 1: Structural Formula for Tofersen
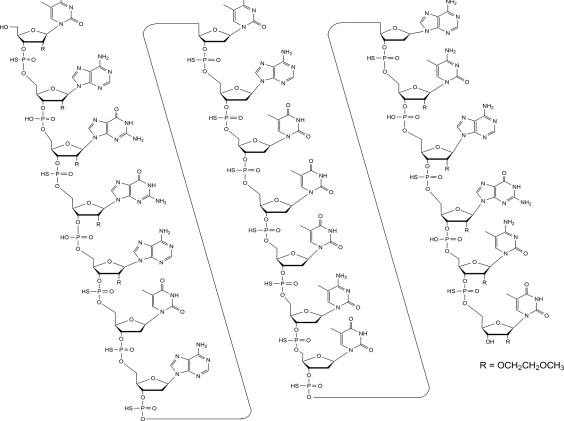
The molecular formula is C
QALSODY is supplied as a sterile, preservative-free, clear, and colorless to slightly yellow solution in a Type I glass vial to be administered by intrathecal administration. Each vial of drug product contains a single dose of 100 mg tofersen at a concentration of 6.7 mg/mL in a formulation containing 0.21 mg/mL calcium chloride dihydrate, 0.11 mg/mL dibasic sodium phosphate, 0.16 mg/mL magnesium chloride hexahydrate, 0.03 mg/mL monobasic sodium phosphate, 0.22 mg/mL potassium chloride, 8.77 mg/mL sodium chloride, and water for injection. The pH of QALSODY is approximately 7.2 (range 6.7 to 7.7).
6CLINICAL STUDIES
The efficacy of QALSODY was assessed in a 28-week randomized, double-blind, placebocontrolled clinical study in patients 23 to 78 years of age with weakness attributable to ALS and a SOD1 mutation confirmed by a central laboratory (Study 1 Part C, NCT02623699). One hundred eight (108) patients were randomized 2:1 to receive treatment with either QALSODY 100 mg (n = 72) or placebo (n = 36) for 24 weeks (3 loading doses followed by 5 maintenance doses). Concomitant riluzole and/or edaravone use was permitted for patients.
The prespecified primary analysis population (n = 60, modified intent to treat [mITT]) had a slow vital capacity (SVC) ≥ 65% of predicted value and met prognostic enrichment criteria for rapid disease progression, defined based on their pre-randomization ALS Functional Rating Scale–Revised (ALSFRS-R) decline slope and SOD1 mutation type.
The non-mITT population (n = 48) had a slow vital capacity (SVC) ≥ 50% of predicted value and did not meet the enrichment criteria for rapid disease progression.
Baseline disease characteristics in the overall intent-to-treat (ITT) population (combined mITT and non-mITT population) were generally similar in patients treated with QALSODY and patients who received placebo, with slightly shorter time from symptom onset and higher plasma NfL at baseline in the QALSODY group. At baseline, 62% of patients were taking riluzole, and 8% of patients were taking edaravone. Mean baseline ALSFRS-R score was 36.9 (5.9) in the QALSODY treatment group and 37.3 (5.8) in the placebo group. Median time from symptom onset was 11.4 months in the QALSODY treatment group and 14.6 months in the placebo group.
The primary efficacy analysis was the change from baseline to Week 28 in the ALSFRS-R total score in the mITT population, analyzed using the joint rank test to account for mortality in conjunction with multiple imputation (MI) to account for missing data for withdrawals other than death. Patients treated with QALSODY experienced less decline from baseline in the ALSFRS-R compared to placebo, but the results were not statistically significant (QALSODY-placebo adjusted mean difference [95% CI]: 1.2 [-3.2, 5.5]). Other clinical secondary outcomes also did not reach statistical significance.
Secondary endpoints of change from baseline at Week 28 in plasma NfL and CSF SOD1 protein were nominally statistically significant (see
Figure 2: Plasma NfL Adjusted Geometric Mean Ratio to Baseline Values in Study 1 Part C by Study Week for the ITT Population
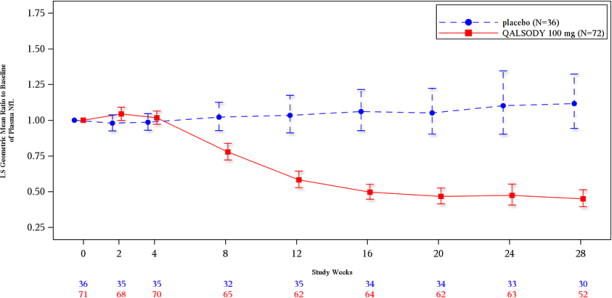
After completion of Study 1, patients had the option to enroll in an open-label extension study. At an interim analysis at 52 weeks, reductions in NfL were seen in patients previously receiving placebo who initiated QALSODY in the open-label extension study, similar to the reductions seen in patients treated with QALSODY in Study 1. Earlier initiation of QALSODY compared to placebo/delayed initiation of QALSODY was associated with trends for reduction in decline on ALSFRS-R, SVC percent-predicted, and hand-held dynamometry (HHD) megascore that were not statistically significant. Through all open-label follow-up at the time of the interim analysis, earlier initiation of QALSODY was also associated with a trend towards reduction of the risk of death or permanent ventilation, although it was not statistically significant. These exploratory analyses should be interpreted with caution given the limitations of data collected outside of a controlled study, which may be subject to confounding.