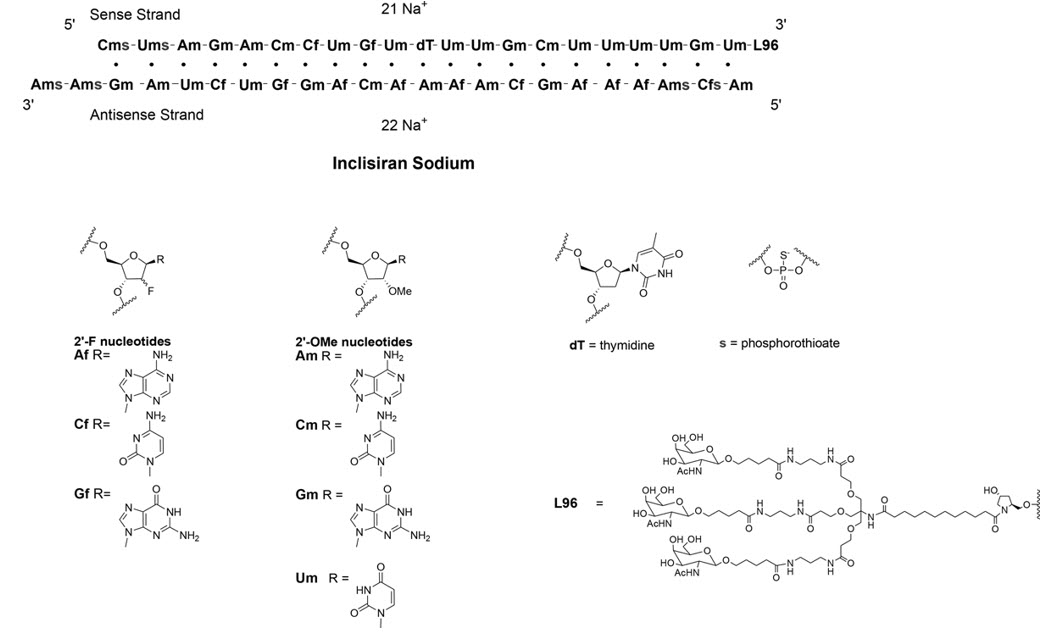
The efficacy of LEQVIO was investigated in three randomized, double-blind, placebo-controlled trials that enrolled 3,660 adults with HeFH, clinical ASCVD, or increased risk for ASCVD, who were taking maximally tolerated statin therapy and who required additional LDL-C lowering. Demographics and baseline disease characteristics were balanced between the treatment arms in all trials.
Primary Hypercholesterolemia
Study 1 (ORION-10, NCT03399370) was a multicenter, double-blind, randomized, placebo-controlled 18-month trial in which 1,561 patients with ASCVD were randomized 1:1 to receive subcutaneous injections of either LEQVIO 284 mg (n = 781) or placebo (n = 780) on Day 1, Day 90, Day 270, and at Day 450. Patients were taking a maximally tolerated dose of statin with or without other lipid modifying therapy, and required additional LDL-C reduction. Patients were stratified by current use of statins or other lipid-modifying therapies. Patients taking PCSK9 inhibitors were excluded from the trial.
The mean age at baseline was 66 years (range: 35 to 90 years), 60% were ≥65 years old, 31% were women, 86% were White, 13% were Black or African American, 1% were Asian, and 14% identified as Hispanic or Latino ethnicity. Forty-five percent (45%) of patients had diabetes at baseline. The mean baseline LDL-C was 105 mg/dL. At the time of randomization, 89% of patients were receiving statin therapy and 69% were receiving high-intensity statin therapy.
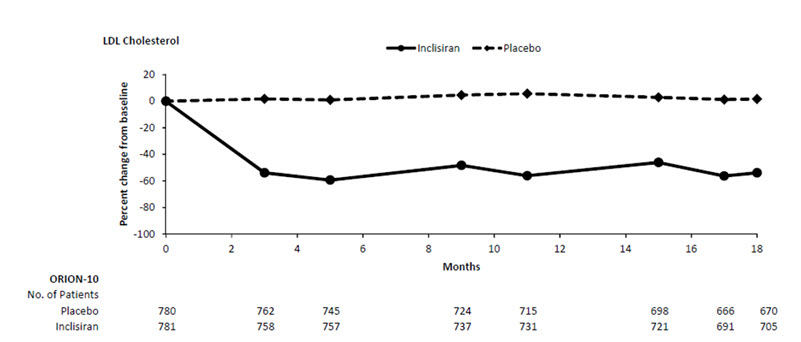
The primary efficacy outcome measure in Study 1 was the percent change from baseline to Day 510 in LDL-C. The difference between the LEQVIO and placebo groups in mean percentage change in LDL-C from baseline to Day 510 was -52% (95% CI: -56%, -49%; p < 0.0001). For additional results, see Table 2 and Figure 1.
Figure 1: Mean Percent Change from Baseline in LDL-C Over 18 Months in Patients with Hypercholesterolemia and ASCVD on Maximally Tolerated Statin Therapy (Study 1)
Study 2 (ORION-11, NCT03400800) was a multicenter, double-blind, randomized, placebo-controlled 18-month trial in which 1,617 adults with ASCVD or increased risk for ASCVD were randomized 1:1 to receive subcutaneous injections of either LEQVIO 284 mg (n = 810) or placebo (n = 807) on Day 1, Day 90, Day 270, and Day 450. Patients were taking a maximally tolerated dose of statin with or without other lipid modifying therapy and required additional LDL-C reduction. Patients were stratified by country and by current use of statins or other lipid-modifying therapies. Patients taking PCSK9 inhibitors were excluded from the trial.
The mean age at baseline was 65 years (range: 20 to 88 years), 55% were ≥65 years old, 28% were women, 98% were White, 1% were Black or African American, and <1% were Asian; <1% identified as Hispanic or Latino ethnicity. Thirty-five percent (35%) of patients had diabetes at baseline. The mean baseline LDL-C was 105 mg/dL. At the time of randomization, 95% of patients were receiving statin therapy and 78% were receiving high-intensity statin therapy.
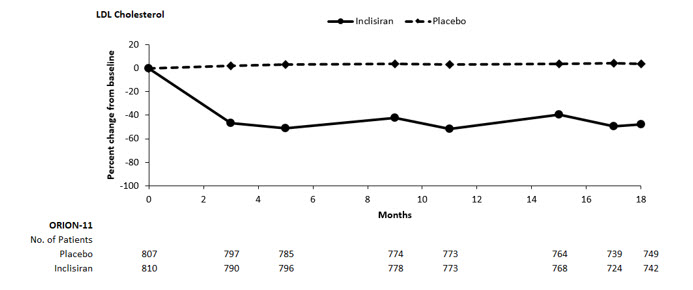
The primary efficacy outcome measure in Study 2 was the percent change from baseline to Day 510 in LDL-C. The difference between the LEQVIO and placebo groups in mean percentage change in LDL-C from baseline to Day 510 was -50% (95% CI: -53%, -47%; p < 0.0001). For additional results, see Table 3 and Figure 2.
Figure 2: Mean Percent Change from Baseline in LDL-C Over 18 Months in Patients with Hypercholesterolemia and ASCVD or Increased Risk for ASCVD on Maximally Tolerated Statin Therapy (Study 2)
In a pooled analysis of Study 1 and Study 2, the observed treatment effect was similar across predefined subgroups, such as sex, age, race, disease characteristics, geographic regions, presence of diabetes, body mass index, baseline LDL-C levels, and intensity of statin treatment.
LDL-C Reduction in Patients with HeFH
Study 3 (ORION-9, NCT03397121) was a multicenter, double-blind, randomized, placebo-controlled 18-month trial in which 482 patients with HeFH were randomized 1:1 to receive subcutaneous injections of either LEQVIO 284 mg (n = 242) or placebo (n = 240) on Day 1, Day 90, Day 270, and at Day 450. Patients with HeFH were taking a maximally tolerated dose of statin with or without other lipid modifying therapy and required additional LDL-C reduction. The diagnosis of HeFH was made either by genotyping or clinical criteria using either the Simon Broome or WHO/Dutch Lipid Network criteria. Patients were stratified by country and by current use of statins or other lipid-modifying therapies. Patients taking PCSK9 inhibitors were excluded from the trial.
The mean age at baseline was 55 years (range: 21 to 80 years), 22% were ≥65 years old, 53% were women, 94% were White, 3% were Black or African American, and 3% were Asian; and 3% identified as Hispanic or Latino ethnicity. Ten percent (10%) of patients had diabetes at baseline. The mean baseline LDL-C was 153 mg/dL. At the time of randomization, 90% of patients were receiving statin therapy and 74% were receiving high-intensity statin therapy. Fifty-two percent (52%) of patients were treated with ezetimibe. The most commonly administered statins were atorvastatin and rosuvastatin.
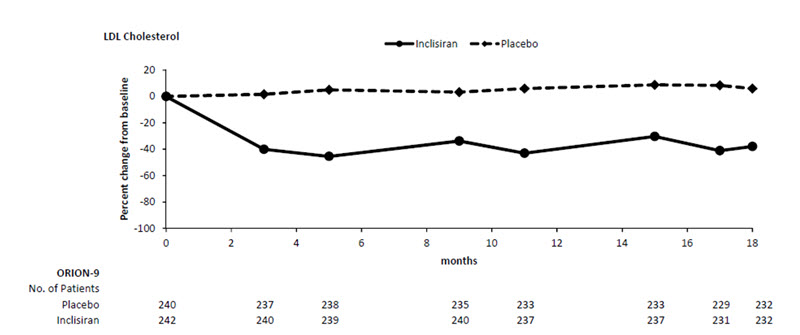
The primary efficacy outcome measure in Study 3 was the percent change from baseline to Day 510 in LDL-C. The difference between the LEQVIO and placebo groups in mean percentage change in LDL-C from baseline to Day 510 was -48% (95% CI: -54%, -42%; p < 0.0001). For additional results, see Table 4 and Figure 3.
Figure 3: Mean Percent Change from Baseline in LDL-C Over 18 Months in Patients with HeFH on Maximally Tolerated Statin Therapy (Study 3)