Generic Name
Roflumilast
Brand Names
Zoryve, Daliresp
FDA approval date: July 01, 2015
Classification: Phosphodiesterase 4 Inhibitor
Form: Cream, Aerosol, Tablet
What is Zoryve (Roflumilast)?
Roflumilast is a selective phosphodiesterase 4 inhibitor indicated as a treatment to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. Limitations of Use: Roflumilast is not a bronchodilator and is not indicated for the relief of acute bronchospasm. Roflumilast tablets 250 mcg is a starting dose, for the first 4 weeks of treatment only and is not the effective dose. Roflumilast tablets are indicated as a treatment to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. Limitations of Use Roflumilast is not a bronchodilator and is not indicated for the relief of acute bronchospasm. Roflumilast tablet 250 mcg is a starting dose, for the first 4 weeks of treatment only and is not the effective dose.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ZORYVE (roflumilast)
1DOSAGE AND ADMINISTRATION
Plaque Psoriasis
- Use ZORYVE cream, 0.3%, for the treatment of plaque psoriasis in adult and pediatric patients 6 years of age and older.
Atopic Dermatitis
- Use ZORYVE cream, 0.15%, for the treatment of mild to moderate atopic dermatitis in adult and pediatric patients 6 years of age and older.
- Use ZORYVE cream, 0.05%, for the treatment of mild to moderate atopic dermatitis in pediatric patients 2 to 5 years of age.
Administration Instructions
- Apply ZORYVE cream to affected areas once daily and rub in completely. Wash hands after application.
- ZORYVE cream is for topical use only and not for ophthalmic, oral, or intravaginal use.
2DOSAGE FORMS AND STRENGTHS
- Cream, 0.3%: 3 mg of roflumilast per gram of white to off-white cream in 60-gram tubes.
- Cream, 0.15%: 1.5 mg of roflumilast per gram of white to off-white cream in 60-gram tubes.
- Cream, 0.05%: 0.5 mg of roflumilast per gram of white to off-white cream in 60-gram tubes.
3CONTRAINDICATIONS
ZORYVE cream is contraindicated in patients with moderate to severe liver impairment (Child-Pugh B or C)
4DESCRIPTION
ZORYVE (roflumilast) cream is a white to off-white cream for topical use. The active ingredient, roflumilast, is a phosphodiesterase 4 (PDE4) inhibitor.
Roflumilast is described chemically as 3-cyclopropylmethoxy-
The structural formula is represented below:
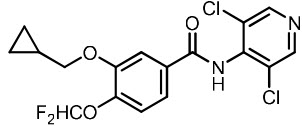
Roflumilast is practically insoluble in water and hexane, sparingly soluble in ethanol, and freely soluble in acetone.
Each gram of cream, 0.05%, 0.15%, or 0.3%, contains 0.5 mg, 1.5 mg, or 3 mg of roflumilast, respectively, in a cream base containing ceteareth-10 phosphate, cetearyl phosphate, cetostearyl alcohol, diethylene glycol monoethyl ether, hexylene glycol, isopropyl palmitate, methylparaben, propylparaben, purified water, sodium hydroxide, and white petrolatum. Hydrochloric acid may have been added to adjust pH.
5HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ZORYVE (roflumilast) cream is a white to off-white cream supplied as follows:
- Cream, 0.3%: 3 mg of roflumilast per gram supplied in 60-gram aluminum tubes (NDC 80610-130-60).
- Cream, 0.15%: 1.5 mg of roflumilast per gram supplied in 60-gram aluminum tubes (NDC 80610-115-60).
- Cream, 0.05%: 0.5 mg of roflumilast per gram supplied in 60-gram aluminum tubes (NDC 80610-105-60).
6PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information).
Administration Instructions
Advise patients or caregivers that ZORYVE cream is for topical use only and is not for ophthalmic, oral, or intravaginal use.
Instruct patients and caregivers to wash hands after applying ZORYVE cream.
Lactation
Advise the patient to use ZORYVE cream on the smallest area of skin and for the shortest duration possible while breastfeeding. Instruct the patient who is breastfeeding not to apply ZORYVE cream directly to the nipple or areola to avoid direct infant exposure. Instruct the patient to avoid inadvertent contact of treated areas with infant skin
7PRINCIPAL DISPLAY PANEL - 60 g Tube Carton - NDC 80610-130-60
NDC 80610-130-60
ZORYVE
For Topical Use Only
Rx Only
ARCUTIS
8PRINCIPAL DISPLAY PANEL - 60 g Tube Carton - NDC 80610-115-60
NDC 80610-115-60
ZORYVE
For Topical Use Only
Rx Only
ARCUTIS

9PRINCIPAL DISPLAY PANEL - 60 g Tube Carton - NDC 80610-105-60
NDC 80610-105-60
ZORYVE
For Topical Use Only
Rx Only
ARCUTIS
