Generic Name
Tiotropium
Brand Names
Spiriva HandiHaler, Spiriva Respimat
FDA approval date: October 11, 2005
Classification: Anticholinergic
Form: Spray, Capsule
What is Spiriva HandiHaler (Tiotropium)?
SPIRIVA HANDIHALER is indicated for the long-term, once-daily, maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease , including chronic bronchitis and emphysema. SPIRIVA HANDIHALER is indicated to reduce exacerbations in COPD patients. SPIRIVA HANDIHALER is an anticholinergic indicated for the long-term, once-daily, maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease , and for reducing COPD exacerbations
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Spiriva (TIOTROPIUM BROMIDE)
1INDICATIONS AND USAGE
SPIRIVA HANDIHALER (tiotropium bromide inhalation powder) is indicated for the long-term, once-daily, maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. SPIRIVA HANDIHALER is indicated to reduce exacerbations in COPD patients.
2DOSAGE AND ADMINISTRATION
For oral inhalation only. Do not swallow SPIRIVA capsules, as the intended effects on the lungs will not be obtained. The contents of the SPIRIVA capsules should only be used with the HANDIHALER device [see .
The recommended dosage of SPIRIVA HANDIHALER is two inhalations of the powder contents of one SPIRIVA capsule, once-daily, with the HANDIHALER device
For administration of SPIRIVA HANDIHALER, a SPIRIVA capsule is placed into the center chamber of the HANDIHALER device. The SPIRIVA capsule is pierced by pressing and releasing the green piercing button on the side of the HANDIHALER device. The tiotropium formulation is dispersed into the air stream when the patient inhales through the mouthpiece
No dosage adjustment is required for geriatric, hepatically-impaired, or renally-impaired patients. However, patients with moderate to severe renal impairment given SPIRIVA HANDIHALER should be monitored closely for anticholinergic effects
3DOSAGE FORMS AND STRENGTHS
Inhalation Powder: SPIRIVA HANDIHALER consists of SPIRIVA capsules containing tiotropium powder for oral inhalation and a HANDIHALER device. SPIRIVA capsules contain 18 mcg of tiotropium (equivalent to 22.5 mcg tiotropium bromide monohydrate) in a light green, hard gelatin capsule with "TI 01" printed on one side and Boehringer Ingelheim company symbol on the other side. The HANDIHALER device is only intended for use with the SPIRIVA capsules.
4CONTRAINDICATIONS
SPIRIVA HANDIHALER is contraindicated in patients with a hypersensitivity to tiotropium, ipratropium, or any components of this product
5ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections:
- Immediate hypersensitivity reactions
- Paradoxical bronchospasm
- Worsening of narrow-angle glaucoma
- Worsening of urinary retention
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the incidence of adverse reactions observed in the clinical trials of a drug cannot be directly compared to the incidences in the clinical trials of another drug and may not reflect the incidences observed in practice.
5.2Postmarketing Experience
Adverse reactions have been identified during worldwide post-approval use of SPIRIVA HANDIHALER. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions are: application site irritation (glossitis, mouth ulceration, and pharyngolaryngeal pain), dizziness, dysphagia, hoarseness, intestinal obstruction including ileus paralytic, intraocular pressure increased, oral candidiasis, palpitations, pruritus, tachycardia, throat irritation, and urticaria.
6OVERDOSAGE
High doses of tiotropium may lead to anticholinergic signs and symptoms. However, there were no systemic anticholinergic adverse effects following a single inhaled dose of up to 282 mcg tiotropium in 6 healthy volunteers. In a study of 12 healthy volunteers, bilateral conjunctivitis and dry mouth were seen following repeated once-daily inhalation of 141 mcg of tiotropium.
Treatment of overdosage consists of discontinuation of SPIRIVA HANDIHALER together with institution of appropriate symptomatic and/or supportive therapy.
7DESCRIPTION
SPIRIVA HANDIHALER consists of SPIRIVA capsules and a HANDIHALER device. Each light green, hard gelatin SPIRIVA capsule contains a dry powder consisting of 18 mcg tiotropium (equivalent to 22.5 mcg tiotropium bromide monohydrate) blended with lactose monohydrate (which may contain milk proteins).
The contents of SPIRIVA capsules are intended for oral inhalation only, and are intended for administration only with the HANDIHALER device.
The active component of SPIRIVA HANDIHALER is tiotropium. The drug substance, tiotropium bromide monohydrate, is an anticholinergic with specificity for muscarinic receptors. It is chemically described as (1α, 2β, 4β, 5α, 7β)-7-[(Hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.0
The structural formula is:
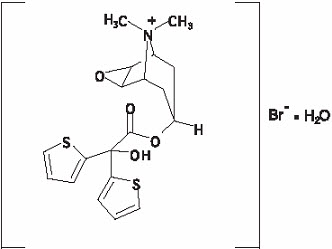
Tiotropium bromide (monohydrate) has a molecular mass of 490.4 and a molecular formula of C
The HANDIHALER device is an inhalation device used to inhale the dry powder contained in the SPIRIVA capsule. The dry powder is delivered from the HANDIHALER device at flow rates as low as 20 L/min. Under standardized
8CLINICAL STUDIES
The SPIRIVA HANDIHALER (tiotropium bromide inhalation powder) clinical development program consisted of six Phase 3 studies in 2,663 patients with COPD (1,308 receiving SPIRIVA HANDIHALER): two 1-year, placebo-controlled studies, two 6-month, placebo-controlled studies and two 1-year, ipratropium-controlled studies. These studies enrolled patients who had a clinical diagnosis of COPD, were 40 years of age or older, had a history of smoking greater than 10 pack-years, had a forced expiratory volume in one second (FEV
In these studies, SPIRIVA HANDIHALER, administered once-daily in the morning, provided improvement in lung function (FEV
Two additional trials evaluated exacerbations: a 6-month, randomized, double-blind, placebo-controlled, multicenter clinical trial of 1,829 COPD patients in a US Veterans Affairs setting and a 4-year, randomized, double-blind, placebo-controlled, multicenter, clinical trial of 5,992 COPD patients. Long-term effects on lung function and other outcomes, were also evaluated in the 4-year multicenter trial.
9HOW SUPPLIED/STORAGE AND HANDLING
SPIRIVA HANDIHALER consists of SPIRIVA capsules and the HANDIHALER device. SPIRIVA capsules contain 18 mcg of tiotropium (equivalent to 22.5 mcg tiotropium bromide monohydrate) and are light green, with the Boehringer Ingelheim company symbol on the SPIRIVA capsule cap and "TI 01" on the SPIRIVA capsule body, or vice versa.
The HANDIHALER device is gray colored with a green piercing button. It is imprinted with SPIRIVA
SPIRIVA capsules are packaged in an aluminum/aluminum blister card and joined along a perforated-cut line. SPIRIVA capsules should always be stored in the blister and only removed immediately before use. The drug should be used immediately after the packaging over an individual SPIRIVA capsule is opened.
The following packages are available:
- carton containing 5 SPIRIVA capsules (1 unit-dose blister card) and 1 HANDIHALER inhalation device (NDC 0597-0075-75) (institutional pack)
- carton containing 30 SPIRIVA capsules (3 unit-dose blister cards) and 1 HANDIHALER inhalation device (NDC 0597-0075-41)
- carton containing 90 SPIRIVA capsules (9 unit-dose blister cards) and 1 HANDIHALER inhalation device (NDC 0597-0075-47)
Keep out of reach of children. Do not get powder into eyes.
10PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
11Patient Information
SPIRIVA
Important Information: Do not swallow SPIRIVA capsules. SPIRIVA capsules should only be used with the HANDIHALER device and inhaled through your mouth (oral inhalation).
Read the information that comes with your SPIRIVA HANDIHALER before you start using it and each time you refill your prescription. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is SPIRIVA HANDIHALER?
- SPIRIVA HANDIHALER is a prescription medicine used each day (a maintenance medicine) to control symptoms of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema.
- SPIRIVA HANDIHALER helps make your lungs work better for 24 hours. SPIRIVA HANDIHALER relaxes your airways and helps keep them open. You may start to feel like it is easier to breathe on the first day, but it may take longer for you to feel the full effects of the medicine. SPIRIVA HANDIHALER works best and may help make it easier to breathe when you use it every day.
- SPIRIVA HANDIHALER reduces the likelihood of flare-ups and worsening of COPD symptoms (COPD exacerbations). A COPD exacerbation is defined as an increase or new onset of more than one COPD symptom such as cough, mucus, shortness of breath, and wheezing that requires medicine beyond your rescue medicine.
SPIRIVA HANDIHALER is not a rescue medicine and should not be used for treating sudden breathing problems. Your doctor may give you other medicine to use for sudden breathing problems.
It is not known if SPIRIVA HANDIHALER is safe and effective in children.
Who should not take SPIRIVA HANDIHALER?
Do not use SPIRIVA HANDIHALER if you:
- are allergic to tiotropium, ipratropium (Atrovent
Symptoms of a serious allergic reaction to SPIRIVA HANDIHALER may include:
- raised red patches on your skin (hives)
- itching
- rash
- swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing
If you have these symptoms of an allergic reaction, stop taking SPIRIVA HANDIHALER and call your doctor right away or go to the nearest hospital emergency room.
What should I tell my doctor before using SPIRIVA HANDIHALER?
Before taking SPIRIVA HANDIHALER, tell your doctor about all your medical conditions, including if you:
- have kidney problems.
- have glaucoma. SPIRIVA HANDIHALER may make your glaucoma worse.
- have an enlarged prostate, problems passing urine, or a blockage in your bladder. SPIRIVA HANDIHALER may make these problems worse.
- are pregnant or plan to become pregnant. It is not known if SPIRIVA HANDIHALER could harm your unborn baby.
- are breast-feeding or plan to breast-feed. It is not known if SPIRIVA HANDIHALER passes into breast milk. You and your doctor will decide if SPIRIVA HANDIHALER is right for you while you breast-feed.
- have a severe allergy to milk proteins. Ask your doctor if you are not sure.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines and eye drops, vitamins, and herbal supplements. Some of your other medicines or supplements may affect the way SPIRIVA HANDIHALER works. SPIRIVA HANDIHALER is an anticholinergic medicine. You should not take other anticholinergic medicines while using SPIRIVA HANDIHALER, including ipratropium. Ask your doctor or pharmacist if you are not sure if one of your medicines is an anticholinergic.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist when you get a new medicine.
How should I take SPIRIVA HANDIHALER?
- Use SPIRIVA HANDIHALER exactly as prescribed. Use SPIRIVA HANDIHALER one time every day.
- Read the "Instructions for Use" at the end of this leaflet before you use SPIRIVA HANDIHALER. Talk with your doctor if you do not understand the instructions.
- Do not swallow SPIRIVA capsules.
- Only use SPIRIVA capsules with the HANDIHALER device.
- Do not use the HANDIHALER device to take any other medicine.
- SPIRIVA HANDIHALER comes as a powder in a SPIRIVA capsule that fits the HANDIHALER device. Each SPIRIVA capsule, containing only a small amount of SPIRIVA powder, is one full dose of medicine.
- Separate one blister from the blister card. Then take out one of the SPIRIVA capsules from the blister package right before you use it.
- After the capsule is pierced, take a complete dose of SPIRIVA HANDIHALER by breathing in the powder by mouth two times, using the HANDIHALER device (take 2 inhalations from one SPIRIVA capsule). See the "
- Throw away any SPIRIVA capsule that is not used right away after it is taken out of the blister package. Do not leave the SPIRIVA capsules open to air; they may not work as well.
- If you miss a dose, take it as soon as you remember. Do not use SPIRIVA HANDIHALER more than one time every 24 hours.
- If you use more than your prescribed dose of SPIRIVA HANDIHALER, call your doctor or a poison control center.
What should I avoid while using SPIRIVA HANDIHALER?
- Do not let the powder from the SPIRIVA capsule get into your eyes. Your vision may get blurry and the pupil in your eye may get larger (dilate). If this happens, call your doctor.
- SPIRIVA HANDIHALER can cause dizziness and blurred vision. Should you experience these symptoms you should use caution when engaging in activities such as driving a car or operating appliances or other machines.
What are the possible side effects of SPIRIVA HANDIHALER?
SPIRIVA HANDIHALER can cause serious side effects, including: Allergic reaction. Symptoms may include:
- raised red patches on your skin (hives)
- itching
- rash
- swelling of the lips, tongue, or throat that may cause difficulty in breathing or swallowing
If you have these symptoms of an allergic reaction, stop taking SPIRIVA HANDIHALER and call your doctor right away or go to the nearest hospital emergency room.
- Sudden narrowing and blockage of the airways into the lungs (bronchospasm). Your breathing suddenly gets worse.
If you have these symptoms of bronchospasm, stop taking SPIRIVA HANDIHALER and call your doctor right away or go to the nearest hospital emergency room.
- New or worsened increased pressure in the eyes (acute narrow-angle glaucoma). Symptoms of acute narrow-angle glaucoma may include:
- eye pain
- blurred vision
- seeing halos (visual halos) or colored images along with red eyes
Using only eye drops to treat these symptoms may not work. If you have these symptoms, stop taking SPIRIVA HANDIHALER and call your doctor right away.
- New or worsened urinary retention. Symptoms of blockage in your bladder and/or enlarged prostate may include: difficulty passing urine, painful urination.
If you have these symptoms of urinary retention, stop taking SPIRIVA HANDIHALER and call your doctor right away.
Other side effects with SPIRIVA HANDIHALER include:
- upper respiratory tract infection
- dry mouth
- sinus infection
- sore throat
- non-specific chest pain
- urinary tract infection
- indigestion
- runny nose
- constipation
- increased heart rate
- blurred vision
These are not all the possible side effects with SPIRIVA HANDIHALER. Tell your doctor if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store SPIRIVA HANDIHALER?
- Do not store SPIRIVA capsules in the HANDIHALER device.
- Store SPIRIVA capsules in the sealed blister package at room temperature 68°F to 77°F (20°C to 25°C).
- Keep SPIRIVA capsules away from heat and cold (do not freeze).
- Store SPIRIVA capsules in a dry place. Throw away any unused SPIRIVA capsules that have been open to air.
Ask your doctor or pharmacist if you have any questions about storing your SPIRIVA capsules.
Keep SPIRIVA HANDIHALER, SPIRIVA capsules, and all medicines out of the reach of children.
General information about SPIRIVA HANDIHALER
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use SPIRIVA HANDIHALER for a purpose for which it has not been prescribed. Do not give SPIRIVA HANDIHALER to other people even if they have the same symptoms that you have. It may harm them.
For more information about SPIRIVA HANDIHALER, talk with your doctor. You can ask your doctor or pharmacist for information about SPIRIVA HANDIHALER that is written for health professionals.
For current prescribing information for SPIRIVA HANDIHALER, scan the code or for additional information you may also call Boehringer Ingelheim Pharmaceuticals, Inc., at 1-800-542-6257.

What are the ingredients in SPIRIVA HANDIHALER?
Active ingredient: tiotropium
What is COPD (Chronic Obstructive Pulmonary Disease)?
COPD is a serious lung disease that includes chronic bronchitis, emphysema, or both. Most COPD is caused by smoking. When you have COPD, your airways become narrow. So, air moves out of your lungs more slowly. This makes it hard to breathe.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Licensed from:
SPIRIVA
Copyright © 2024 Boehringer Ingelheim International GmbH
Revised: December 2024
COL10494BL302024
12Instructions for Use
SPIRIVA® (speh REE vah) HANDIHALER®
Important Information about using your SPIRIVA HANDIHALER
- Do not swallow SPIRIVA capsules.
- SPIRIVA capsules should only be used with the HANDIHALER device and inhaled through your mouth (oral inhalation).
- Do not use your HANDIHALER device to take any other medicine.
First read the Patient Information, then read these Instructions for Use before you start to use SPIRIVA HANDIHALER and each time you refill your prescription. There may be new information.
Becoming familiar with your HANDIHALER device and SPIRIVA capsules:
Your SPIRIVA HANDIHALER comes with SPIRIVA capsules in blister packaging and a HANDIHALER device. Use the new HANDIHALER device provided with your medicine.
Taking your full daily dose of medicine requires 4 main steps.
Step 1. Opening your HANDIHALER device:
Step 2. Inserting the SPIRIVA capsule into your HANDIHALER device:
Step 3. Piercing the SPIRIVA capsule:
Step 4. Taking your full daily dose (2 inhalations from the same SPIRIVA capsule):
Caring for and storing your SPIRIVA HANDIHALER:
If you do not hear or feel the SPIRIVA capsule rattle as you breathe in your medicine:
Cleaning your HANDIHALER device:
For current prescribing information for SPIRIVA HANDIHALER, scan the code or for additional information you may also call Boehringer Ingelheim Pharmaceuticals, Inc., at 1-800-542-6257.

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Licensed from:
SPIRIVA® and HANDIHALER® are registered trademarks of and are used under license from Boehringer Ingelheim International GmbH.
Copyright © 2024 Boehringer Ingelheim International GmbH
Revised: December 2024
COL10494BL302024
13PRINCIPAL DISPLAY PANEL - 30 Capsule Blister Pack Carton
NDC 0597-0075-41
SPIRIVA
18 mcg/Capsule*
DO NOT SWALLOW SPIRIVA CAPSULES.
FOR ORAL INHALATION ONLY
Rx only
SPIRIVA capsules should only
Boehringer
