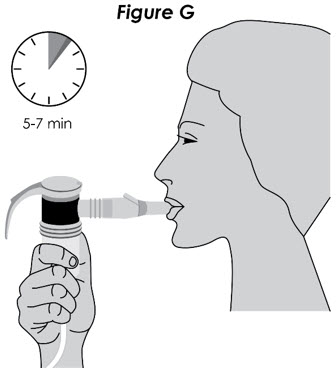
Ohtuvayre
What is Ohtuvayre (Ensifentrine)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study is a randomized, double-blind, placebo-controlled study designed to assess the efficacy and safety of ensifentrine inhalation suspension (3 mg) delivered twice daily via standard jet nebulizer over at least 24 weeks, compared to placebo, in subjects with non-cystic fibrosis bronchiectasis (NCFBE).
Summary: This study will assess the safety and efficacy of fixed dose combinations of ensifentrine with two different glycopyrrolate dose levels compared to placebo and to the individual components of the fixed dose combinations, each administered twice a day via standard jet nebulizer, in adult subjects with chronic obstructive pulmonary disease (COPD).
Summary: This is a randomized, double-blind, placebo-controlled, two-period cross-over study of nebulized ensifentrine (3 mg) or placebo administered BID for two 8-week Treatment Periods. All participants with receive both ensifentrine and placebo during participation. There are 7 in-clinic visits over a total duration of up to 24 weeks participation.
Related Latest Advances
Brand Information
- Paradoxical Bronchospasm
- Psychiatric Events Including Suicidality
- Carton of 60: 60 pouches of 1 unit-dose ampule (NDC 83034-003-60)
- Carton of 60: 12 pouches of 5 unit-dose ampules (NDC 83034-003-65)