Brand Name
Livmarli
Generic Name
Maralixibat
View Brand Information FDA approval date: September 29, 2021
Classification: Ileal Bile Acid Transporter Inhibitor
Form: Tablet, Solution
What is Livmarli (Maralixibat)?
LIVMARLI is an ileal bile acid transporter inhibitor indicated for: the treatment of cholestatic pruritus in patients 3 months of age and older with Alagille syndrome .
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Randomized Double-Blind Placebo-Controlled Phase 3 Study to Evaluate the Efficacy and Safety of Maralixibat in the Treatment of Participants With Cholestatic Pruritus
Summary: The purpose of this study is to determine whether the investigational treatment (maralixibat) is safe and effective in pediatric and adult participants who have cholestatic liver disease with pruritus that has been refractory to other therapies, and who have no other treatment options.
Long-Term SafEty and Clinical Outcomes of LivmArli in Patients in the United States (LEAP-US)
Summary: The objective of this 5-year, prospective, observational cohort study is to evaluate the long-term safety and clinical outcomes of patients with Alagille syndrome (ALGS) or Progressive familial intrahepatic cholestasis (PFIC) treated with Livmarli.
Related Latest Advances
Brand Information
Livmarli (maralixibat chloride)
1DOSAGE FORMS AND STRENGTHS
LIVMARLI Oral solution:
- 9.5 mg of maralixibat per mL (for treatment of ALGS) as a clear, colorless to yellow solution.
- 19 mg of maralixibat per mL (for treatment of PFIC) as a clear, colorless to yellow solution.
LIVMARLI Tablets:
- 10 mg: white to off-white round tablets debossed with MRX on one side, 10 on the other side.
- 15 mg: white to off-white modified oval tablets debossed with MRX on one side, 15 on the other side.
- 20 mg: white to off-white round tablets debossed with MRX on one side, 20 on the other side.
- 30 mg: white to off-white round tablets debossed with MRX on one side, 30 on the other side.
2CONTRAINDICATIONS
LIVMARLI is contraindicated in patients with prior or active hepatic decompensation events (e.g., variceal hemorrhage, ascites, hepatic encephalopathy)
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Hepatotoxicity
- Gastrointestinal Adverse Reactions
- Fat Soluble Vitamin (FSV) Deficiency
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
ALGS:
In the Alagille syndrome clinical development program, which includes five clinical studies comprising 86 patients, patients received doses of LIVMARLI up to 760 mcg/kg per day with a median duration of exposure of 32.3 months (range: 0.03 – 60.9 months). In Trial 1, the 4-week placebo control period occurred after 18 weeks of LIVMARLI treatment. In two supportive studies that included long-term open‑label extensions, only 13 weeks of placebo-controlled treatment occurred which evaluated doses lower than 380 mcg/kg/day. The majority of LIVMARLI exposure in the development program occurred without a placebo control in open-label trial extensions.
The most common adverse reactions (≥5%) for ALGS patients treated with LIVMARLI are presented in
Liver Test Abnormalities
Increase in Transaminases
In a pooled analysis of patients with ALGS (N=86) administered LIVMARLI, increases in hepatic transaminases (ALT) were observed. Seven (8.1%) patients discontinued LIVMARLI due to ALT increases. Three (3.5%) patients had a decrease in dose or interruption of LIVMARLI in response to ALT increases. In the majority of cases, the elevations resolved or improved after discontinuation or dose modification of LIVMARLI. In some cases, the elevations resolved or improved without change in LIVMARLI dosing. Increases to more than three times baseline in ALT occurred in 26% of patients treated with LIVMARLI and increases to more than five times baseline occurred in 3%. AST increases to more than three times baseline occurred in 16% of patients treated with LIVMARLI, and an increase to more than five times baseline occurred in one patient. Elevations in transaminases were asymptomatic and not associated with bilirubin elevations or other laboratory abnormalities.
Increases in Bilirubin
Four (4.6%) patients in the pooled analysis experienced bilirubin increases above baseline, and LIVMARLI was subsequently withdrawn in two of these patients, who had elevated bilirubin at baseline.
PFIC:
In Trial 2, which enrolled 93 patients, 47 patients received doses of LIVMARLI up to 570 mcg/kg BID, with a median duration of exposure of 6 months (range: 0.3-6.7 months).
The most common adverse reactions (≥5%) for PFIC patients treated with LIVMARLI at a rate greater than placebo are presented in
One LIVMARLI-treated patient with an event of mild diarrhea discontinued treatment. Treatment interruptions or dose reductions occurred in 3 (6.4%) LIVMARLI-treated patients due to diarrhea or abdominal pain. No placebo-treated subjects discontinued treatment or had dose reductions or interruptions due to diarrhea.
Hepatotoxicity
LIVMARLI treatment is associated with a potential for drug-induced liver injury. In PFIC patients in Trial 2, treatment-emergent elevations of liver tests or worsening of liver tests, relative to baseline values, and hepatic decompensation events were observed. Two patients experienced drug-induced liver injury (DILI) attributable to LIVMARLI; one patient received 570 mcg/kg twice daily and the second patient required dose interruption and reduction. Two additional patients experienced DILI in the open-label extension portion of the trial. Of these four patients, one patient required liver transplant and another patient died.
Biliary Complications
In the placebo-controlled portion of Trial 2, two LIVMARLI-treated patients developed cholangitis or cholecystitis within 3-weeks of drug discontinuation (after 84 days and 130 days after initiating LIVMARLI treatment, respectively). Four LIVMARLI-treated patients developed cholecystitis or cholangitis in the open-label extension portion of Trial 2; the average time to onset was 281 days.
Bone Fracture
In PFIC patients in Trial 2, treatment-emergent bone fracture events were observed. Three receiving LIVMARLI experienced bone fractures relative to none in placebo-treated patients. The median time to onset of fractures was 73 days. Two LIVMARLI-treated patients developed bone fractures in the open-label portion of Trial 2, with average time to onset of fracture was 204 days.
Bleeding
In PFIC patients in Trial 2, treatment-emergent events of hematochezia (4 [8.5%] versus 1 [2.2%]), decrease in hemoglobin greater than or equal to 2 grams/dL from baseline (8 [17%] versus 1 [2.2%]), were reported more frequently in LIVMARLI-treated patients relative to placebo-treated patients
3.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of LIVMARLI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disorders: hematemesis, liver transplant, post-endoscopy hemorrhage, post-liver biopsy hemorrhage
General disorders and administration site conditions: drug ineffective
Injury, poisoning and procedural complications: off label use
Investigations: gamma-glutamyltransferase increased
Nervous system disorders: intracranial hemorrhage
4OVERDOSAGE
Single doses of maralixibat up to 500 mg, approximately 18-fold higher than the recommended dose, have been administered in healthy adults and were tolerated without a meaningful increase in adverse effects when compared to lower doses. If an overdose occurs, discontinue LIVMARLI, monitor the patient for any signs and symptoms and institute general supportive measures if needed.
LIVMARLI contains propylene glycol as an excipient. In cases of suspected overdose, monitor for signs of propylene glycol toxicity, including hemolysis, hyperosmolarity with anion gap metabolic acidosis, acute kidney injury, and CNS toxicity. Discontinue LIVMARLI if propylene glycol toxicity is suspected.
5DESCRIPTION
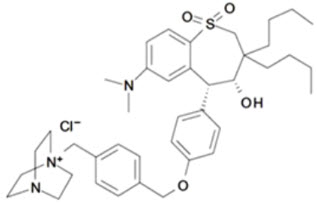
LIVMARLI (maralixibat) is an ileal bile acid transporter (IBAT) inhibitor. Maralixibat is present as a chloride salt with the chemical name 1-[[4-[[4-[(4
LIVMARLI oral solution is supplied in a multiple-dose bottle containing 9.5 mg of maralixibat per mL (equivalent to 10 mg of maralixibat chloride per mL), or containing 19 mg of maralixibat per mL (equivalent to 20 mg of maralixibat chloride per mL). The oral solution contains the following inactive ingredients: edetate disodium, grape flavor, propylene glycol, purified water, and sucralose. The pH of the oral solution is 3.8 – 4.8.
LIVMARLI tablets are available in 10 mg, 15 mg, 20 mg and 30 mg strengths of maralixibat (equivalent to 10.5 mg, 15.8 mg, 21 mg, and 31.6 mg maralixibat chloride, respectively). The tablets contain the following inactive ingredients: crospovidone, glyceryl distearate, lactose monohydrate, microcrystalline cellulose, and silicon dioxide.
6HOW SUPPLIED/STORAGE AND HANDLING
LIVMARLI Oral Solution
LIVMARLI is a clear, colorless to yellow oral solution.
For ALGS: 9.5 mg per mL
- Each amber plastic bottle contains LIVMARLI oral solution at a concentration of 9.5 mg per mL.
- One 30 mL amber plastic bottle: NDC 79378-110-01
For PFIC: 19 mg per mL
- Each amber plastic bottle contains LIVMARLI oral solution at a concentration of 19 mg per mL.
- One 30 mL amber plastic bottle: NDC 79378-111-01
LIVMARLI Tablets
- 10 mg are white to off-white round tablets debossed with MRX on one side, 10 on the other side.
- Bottles of 30 tablets: NDC 79378-210-30
- 15 mg are white to off-white modified oval tablets debossed with MRX on one side, 15 on the other side.
- Bottles of 30 tablets: NDC 79378-215-30
- 20 mg are white to off-white round tablets debossed with MRX on one side, 20 on the other side.
- Bottles of 30 tablets: NDC 79378-220-30
- 30 mg are white to off-white round tablets debossed with MRX on one side, 30 on the other side.
- Bottles of 30 tablets: NDC 79378-230-30
Storage and Handling
Store unopened LIVMARLI oral solution and tablets between 20°C and 25°C (68°F and 77°F), excursion permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Discard any remaining LIVMARLI oral solution 100 days after opening the bottle
7PATIENT COUNSELING INFORMATION
Advise the patient or their caregiver(s) to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Administration Instructions
Advise patients or their caregivers(s) to:
- Take LIVMARLI 30 minutes prior to a meal once or twice daily as prescribed.
- For LIVMARLI oral solution, use a calibrated measuring device (0.5 mL, 1 mL or 3 mL oral dispenser) provided by the pharmacist to measure and deliver the prescribed dose accurately
- Take LIVMARLI at least 4 hours before or 4 hours after taking a bile acid binding resin (e.g., cholestyramine, colesevelam, or colestipol)
- Store the opened LIVMARLI oral solution bottle below 30⁰C (86⁰F). Discard any unused LIVMARLI 100 days after opening the oral solution bottle
Hepatotoxicity
Advise patients or their caregiver(s) that liver tests should be obtained before starting LIVMARLI and periodically during LIVMARLI therapy. Inform patients or their caregiver(s) of the risk of hepatotoxicity that could be fatal and that they will need to undergo monitoring for liver injury. Instruct patients or their caregiver(s) to immediately report any signs or symptoms of severe liver injury to their healthcare provider
Gastrointestinal Adverse Reactions
Advise patients or their caregiver(s) to notify their healthcare provider if they experience a new onset or worsening of gastrointestinal symptoms (abdominal pain, vomiting, bloody stool, and diarrhea)
Fat Soluble Vitamin (FSV) Deficiency
Advise patients or their caregiver(s) that INR (for vitamin K) and serum levels of vitamins A, D, E will be obtained before starting treatment and periodically during treatment to assess for FSV deficiency
Rx only
Manufactured for:
© 2025 Mirum Pharmaceuticals, Inc.
LB00203 / LB00212
8PRINCIPAL DISPLAY PANEL - 9.5 mg/mL Bottle Label
NDC 79378-110-01
Livmarli
9.5 mg/mL
Recommended Dosage:
30 mL

9PRINCIPAL DISPLAY PANEL - 19 mg/mL Bottle Label
NDC 79378-111-01
Livmarli
19 mg/mL
For use in Progressive Familial
30 mL
10PRINCIPAL DISPLAY PANEL – 10 mg Bottle Label
GTIN: (01) 00379378210305; NDC 79378-210-30; Rx only

11PRINCIPAL DISPLAY PANEL – 15 mg Bottle Label
GTIN: (01) 00379378215300; NDC 79378-215-30; Rx only

12PRINCIPAL DISPLAY PANEL – 20 mg Bottle Label
GTIN: (01) 00379378220304; NDC 79378-220-30; Rx only

13PRINCIPAL DISPLAY PANEL – 30 mg Bottle Label
GTIN: (01) 00379378230303; NDC 79378-230-30; Rx only



