Has your doctor recommended a clinical trial? Are you contemplating whether or not to participate in one?
Clinical trials are a type of medical research that studies new tests and treatments to evaluate their effects on human health. The purpose of clinical trials is to find improvements or discoveries for disease diagnoses, treatments, or new approaches to current therapies or treatments.
Some patients are apprehensive because clinical trials are commonly believed to be used only as a last resort for patients facing life-threatening conditions. While patients may participate in a clinical trial as a last resort, researchers conduct these studies for a wide variety of medical scenarios. We talked to two doctors who have clinical trial experience to uncover who clinical trials are for, the associated risks, and what patients can ask their doctors before consenting to participate. We also talked to a patient who participated in a clinical trial.
Are Clinical Trials a Last Resort for Patients?
While clinical trials may provide a glimmer of hope to patients managing life-threatening conditions, many trials focus on newly diagnosed patients or healthy subjects who are willing to test a new drug. There are many different clinical trials for varying types of conditions, medications, treatments, and modalities.
According to Gajendra Singh, MD, a general surgeon practicing in North Carolina, you can enroll in a clinical trial for diseases that either do not have available treatments or new medications that may produce better results for existing conditions and treatments. Dr. Singh says, “Many clinical trials are done for diseases where we do not have definitive treatments or other treatments that are not very successful.”
We talked with Karen Jubanyik, MD, Associate Professor, and attending physician in the Department of Emergency Medicine at Yale New Haven Hospital and Yale University. Dr. Jubanyik has been enrolling patients in clinical trials since 1988 and has served on the Yale Institutional Review Board (IRB), the committee that approves all clinical research on human subjects.
According to Dr. Jubanyik, many clinical trials exist; some for new medications, new devices, new types of surgery, diets, medical procedures, and other interventions.
I am an investigator for a clinical trial where we randomize seriously ill patients to one of two different kinds/modes of palliative care. Some clinical trials might look at whether physical therapy every day is better than three times a week after a particular type of knee surgery (seems pretty benign), while others might be asking patients to try a new COVID vaccination and then walk into a room full of sick COVID patients (not so benign!).
Clinical Trial Phases and “Last Resort” Patients
Each clinical trial has four to five phases of testing. In phase I, researchers assess the amount of a treatment dose that can be safely administered to patients without serious side effects. Since phase I trials carry the most risk, “last resort” patients sometimes choose to join them when they have exhausted all of their options. Phase II test subjects receive the new treatment and continue to be monitored for side effects. In phase III, the experimental treatment is compared against the standard treatment to determine safety and effectiveness. Phase III trials are often longer than phase I and II and may even be conducted at hospitals. After phase III, the FDA may approve the new treatment, at which time it enters phase IV. In phase IV, researchers test the safety and effectiveness of the newly approved drug over time.
Note that some clinical trials also have a phase 0, which closely relates to phase I, and involves giving a sub-therapeutic dose of a drug to 10 to 15 people.

“Last resort” patients are eligible to participate in some phase I clinical trials that test unapproved treatments. According to the Mayo Clinic, “The goals of a phase I clinical trial, also called an early-phase clinical trial, are to determine safe dosage levels and safe methods of delivering a new treatment. An early-phase clinical trial might be the first time an experimental cancer drug or intervention is used with people.”

We also discussed phase I clinical trials with Dr. Jubanyik. She says that the FDA has stringent rules and regulations for approving new drugs and devices. A medication must go through a phase I trial to prove that it is safe and determine its safe dosage. Yet, these trials are often not highly beneficial to patients.
There is often little benefit to the participants in a phase I trial. For cancer chemotherapy, often phase I participants have no reasonable curative treatments left to them. Still, they agree to participate to help others in the future (and might themselves hold out some hope that the new medication might help them, though they are expressly counseled in the Informed Consent process that there should be no expectation that the drug will help them).
Phase I clinical trials are not only attractive to “last resort” patients. Dr. Jubanyik mentioned that some phase I clinical trials may enroll healthy volunteers to test a new medication. Some trials that investigate a disease process look for evidence of its presence in blood tests or imaging. This will require enrolling healthy control subjects.
For people who want to participate as healthy controls, the benefits are usually knowing that they contribute to the development of information that can help other (sick) people someday. They might also benefit from some money, usually in the form of a gift card, though usually these amounts are kept to a relatively small amount that would not unduly entice people.
It’s also important to note that many years of studies are conducted before a new drug/intervention even gets tested on humans. These preclinical studies that involve in vitro (test tube or cell culture) and in vivo (animal) testing can take 10 to 12 years to complete.
Expanded Access (Compassionate Use)
This topic is quite extensive but we wanted to give it a quick mention so you are aware of your medical options. Expanded access, sometimes called “compassionate use” allows patients access to investigational drugs (not yet approved by the FDA) outside of clinical trials when alternatives are not available. Investigation drugs are offered during clinical trials, but when they are used outside of a trial, the action is labeled “compassionate use.” The FDA offers more information on expanded access here.
What are the Benefits of Participating in a Clinical Trial?
Today’s promising treatments were yesterday’s clinical trials. Though clinical trials may introduce some risk to participants, they are generally safe and can improve healthcare by paving the way for new therapies to enter the medical scene.
Jame Abraham, M.D., U.S. Health News contributor, believes a “scientifically sound – and ethically and expertly designed – clinical trial is the gold standard in treatment. While clinical trials are key in cancer research, they’re also important in a range of diseases and conditions.”

Dr. Singh cited numerous benefits of clinical trials. He says, “They can be beneficial to patients who are currently suffering from diseases from which they cannot get complete relief or there is no cure for the disease.” Dr. Singh also discussed the possibility of participants receiving financial incentives. Patients who can’t afford conventional medicine can receive free treatment, which will continue after the trial ends.
Instead of a last resort, a clinical trial may be the first choice for patients receiving treatments they otherwise would not have access to. Some patients receive early access to promising therapies with the added benefit of not paying for them.
Dr. Jubanyik cites one of the benefits of participating in a clinical trial as obtaining access to a needed or desired treatment that is only available via a trial. She says, “Before a drug is approved in the US, the FDA has to look at data from several different types of trials to decide whether to give the pharmaceutical company permission to manufacture the drug and market it for that indication. If the drug has already been approved for one indication, however, individual providers can prescribe it to patients with other conditions—called off label prescribing —but the drug company cannot market it for the new indication without doing a new FDA study.”
Lynn’s Experience with clinical trials
We talked with Lynn C, a chronic pain patient, and patient advocate, who has volunteered for over two dozen clinical trials. She lives with multiple chronic conditions, such as fibromyalgia, chronic migraine, and Ehlers-Danlos Syndrome, and volunteers for relevant studies. Lynn cites clinical trials as a huge part of her resiliency in walking again after being confined to a wheelchair. She feels a greater sense of purpose and part of something greater than herself.
Some people volunteer for clinical trials in order to try new medications that are not yet available to the public. For the most part, I volunteer for non-medication trials to show the researchers how people’s brains, with one of my conditions, such as fibromyalgia, react to certain stimuli, such as pain. Perhaps, if I’m lucky, I’ll be part of the cure for one of my rare diseases!
We also asked Lynn if she had any adverse reactions during her clinical trials. She did during one particular study involving transcranial magnetic stimulation (TMS), which is a noninvasive procedure that uses magnetic fields to stimulate nerve cells in the brain. As part of the study, she was required to wear electrodes on her head while magnets stimulated her brain. She said, “Unfortunately, I either had an allergic reaction to the paste and/or adverse side effects to the magnets, becoming very nauseous and dizzy. This left me unable to do the next part of the study immediately afterward, which involves walking very quickly on a treadmill.”
This is one risk of participating in clinical trials, and it’s part of the informed consent a participant must give. Adverse events are always a risk, and are in fact one of the main things researchers track with experimental treatments.
The Potential Risks of Joining a Clinical Trial
Patients who join clinical trials may feel like lab rats or “guinea pigs,” but clinical trials are not random lab experiments. According to Dr. Abraham, clinical trials are safe, and participants are not considered “guinea pigs.”
A scientifically sound and ethically designed clinical trial undergoes many checks and balances to protect the patient. Patient safety, from beginning to end, is key to the decision-making for the design of any trial. Therefore, anyone who signs up to participate in a trial can be assured that they are not considered a guinea pig, but rather, part of an exciting new treatment that could change the treatment of that condition forever.

Though clinical trials are generally safe, they still come with risks. According to Dr. Singh, during clinical trials, the side effects, risks, and complications of a new treatment have not yet been verified and documented for humans. The drugs and treatments have been tested on animals, but there may be different side effects for humans not encountered in animal testing. Though Dr. Singh cites the various risks involved, he has never witnessed adverse reactions.
He says, “Various medication and treatment modalities have different side effects and complications. The most common complications are allergic reactions, abnormality in the liver, and kidney functions, but sometimes there can be serious side effects or complications from clotting, bleeding, and many more.”
Dr. Jubanyik has witnessed adverse reactions; however, in many cases, the patients were warned about the risks before they participated.
I have seen some life-threatening reactions to drugs given to patients in clinical trials. Sometimes, these reactions cannot be predicted, but most of the time, these were potential reactions that the patient was told about prior to agreeing to be in the trial. These reactions have to be reported to the FDA, who might even stop a clinical trial if it becomes apparent that the risks outweigh the benefits.
Since phase I trials study a drug to ensure it is first safe for humans before conducting further testing, they carry the most significant threat of unexpected side effects. The earlier the testing phase, the higher the risks may be.
You also risk not receiving any benefit from a clinical trial. Patients may be hopeful about trying a new treatment or medication, which may not produce expected outcomes.
Questions to Ask Your Doctor Before Participating in a Clinical Trial
Before you decide to enroll in a clinical trial, obtain information on the risks, benefits, and alternatives. According to Dr. Jubanyik, patients should ask their doctors the following questions:
- Is it possible that I might get a placebo (non-active drug) as part of the study?
- What happens if the medication/device injures me in the clinical trial? Will the investigator/drug company pay for all needed treatment for the alleged bad reaction?
- What are some of the bad reactions experienced by others who have already enrolled in the trial?
- It may also be reasonable for the patient to ask the provider, “What are you getting by my enrolling in this trial?” In some cases, the Primary Investigator of a study might get a lot of money for each patient that agrees to enroll in the study. In those cases, a patient might be wise to be wary.
Dr. Singh suggests patients ask about the trial and why researchers are conducting the study. He also recommends asking about any complications or documented deaths from previous trials. Also, the treatment and medications given in the trial should be free to the patient.
How to Research Current Clinical Trials
Suppose you want to research past clinical trials to discuss with your doctor. In that case, consult MediFind, a platform that uses cutting-edge machine learning techniques to sift through a massive amount of medical data to provide up-to-date information on clinical trials, studies, and publications on a specific condition.
With MediFind, you can search and review completed clinical trials as well as active ones that you might want to discuss with your doctor. Researchers conduct thousands of clinical trials every year, so if you want to participate in one, don’t be afraid to talk to your doctor as they may not be aware of every trial currently going on in a particular disease space.
Let’s walk through a search on MediFind. If you were diagnosed with Sarcoidosis, you can search MediFind’s platform for this condition, and review the clinical trials presented:
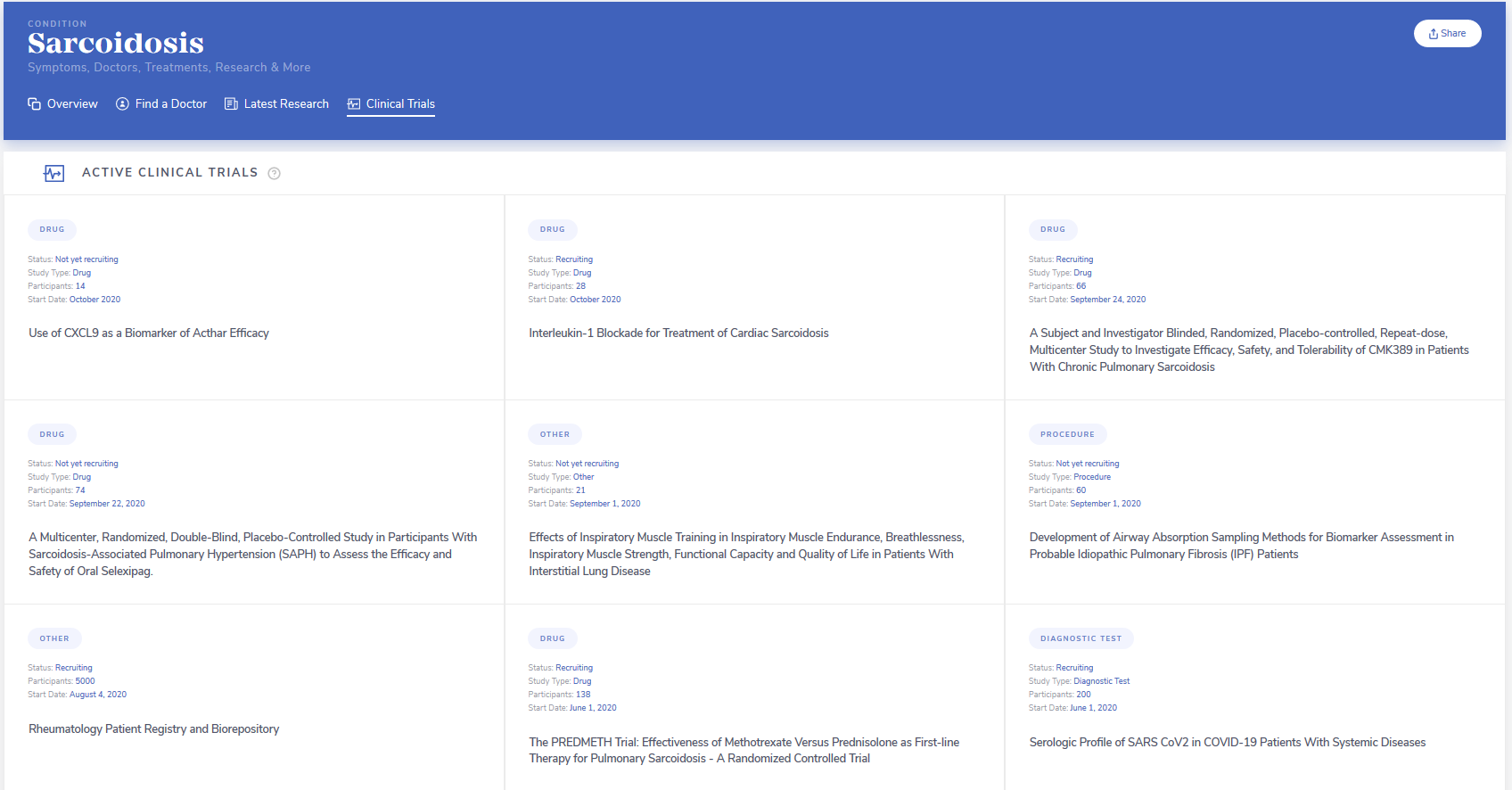
If you are unsure of your condition, MediFind’s Symptom Checker can help you narrow down potential diagnoses to discuss with your doctor. MediFind also enables you to find doctors who are experts in specific conditions.
Clinical trials can be a last resort for patients looking for life-saving treatment after exhausting other options. But most patients who participate in clinical trials are newly diagnosed or healthy and simply looking for new or alternative drug or preventative treatment options.
While clinical trials are generally safe, there is no guarantee that they will improve patient outcomes. Before you agree to participate in a clinical trial, research your options with your doctors, and get information on risks and potential outcomes.



