Brand Name
Oxbryta
Generic Name
Voxelotor
View Brand Information FDA approval date: November 25, 2019
Classification: Hemoglobin S Polymerization Inhibitor
Form: Tablet
What is Oxbryta (Voxelotor)?
OXBRYTA is indicated for the treatment of sickle cell disease in adults and pediatric patients 4 years of age and older. This indication is approved under accelerated approval based on increase in hemoglobin . Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial. OXBRYTA is a hemoglobin S polymerization inhibitor indicated for the treatment of sickle cell disease in adults and pediatric patients 4 years of age and older. This indication is approved under accelerated approval based on increase in hemoglobin . Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
OXBRYTA (Voxelotor)
1INDICATIONS AND USAGE
OXBRYTA is indicated for the treatment of sickle cell disease (SCD) in adults and pediatric patients 4 years of age and older.
This indication is approved under accelerated approval based on increase in hemoglobin (Hb)
2DOSAGE FORMS AND STRENGTHS
Tablets: 300 mg light purple to purple, oval shaped, biconvex, debossed with "G 300" on one side.
Tablets: 500 mg light yellow to yellow, oval shaped, biconvex, debossed with "GBT 500" on one side.
Tablets for oral suspension: 300 mg light yellow to yellow, round shaped, debossed with "300 D" on one side.
3CONTRAINDICATIONS
OXBRYTA is contraindicated in patients with a history of serious drug hypersensitivity reaction to voxelotor or excipients. Clinical manifestations may include generalized rash, urticaria, mild shortness of breath, mild facial swelling, and eosinophilia
4ADVERSE REACTIONS
The following clinically significant adverse reaction is discussed in other sections of the labeling:
- Hypersensitivity Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of OXBRYTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Drug reaction with eosinophilia and systemic symptoms (DRESS), Pruritis, Angioedema (including swelling of eyelid, face edema, lip swelling, and periorbital swelling).
5DESCRIPTION
OXBRYTA contains voxelotor, a hemoglobin S polymerization inhibitor. The chemical name of voxelotor is 2-hydroxy-6-((2-(1-isopropyl-1
The chemical structure of voxelotor is:
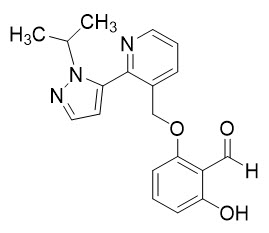
Voxelotor is a white-to-yellow-to-beige compound in crystalline Form II of its free base. It is non-hygroscopic and highly soluble in common organic solvents such as acetone and toluene and insoluble in water.
OXBRYTA film-coated tablets for oral use contain either 300 mg or 500 mg of voxelotor. Both strengths of OXBRYTA film-coated tablets contain the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. In addition, the 500 mg tablet film coating contains: polyethylene glycol 3350, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide. The 300 mg tablet film coating contains: black and red iron oxide, polyethylene glycol 3350, polyvinyl alcohol, talc, and titanium dioxide.
Each OXBRYTA tablet for oral suspension contains 300 mg of voxelotor with the following inactive ingredients: artificial grape flavor, colloidal silicon dioxide, croscarmellose sodium, iron oxide pigment, magnesium stearate, microcrystalline cellulose, and sucralose.
6HOW SUPPLIED/STORAGE AND HANDLING
The 300 mg tablet is film-coated, light purple to purple, oval shaped, biconvex, debossed with "G 300" on one side, available in:
- Bottles of 60 tablets with one desiccant canister, a polyester coil and child-resistant closure: NDC 72786-102-02
- Bottles of 90 tablets with one desiccant canister, a polyester coil and child-resistant closure: NDC 72786-102-03
The 500 mg tablet is film-coated, light yellow to yellow, oval shaped, biconvex, debossed with "GBT 500" on one side, and available in:
- Bottles of 90 tablets with one desiccant canister, a polyester coil and child-resistant closure: NDC 72786-101-01
The 300 mg tablet for oral suspension is light yellow to yellow, round shaped, debossed with "300 D" on one side, and available in:
- Bottles of 60 tablets for oral suspension with a polyester coil and child-resistant closure: NDC 72786-111-02
- Bottles of 90 tablets for oral suspension with a polyester coil and child-resistant closure: NDC 72786-111-03
Do not eat the desiccant canister or the polyester coil.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
8PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
NDC 72786-101-01
Oxbryta
(voxelotor)
500 mg
Swallow tablets whole.
90 tablets
9PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle Label
NDC 72786-111-03
Oxbryta
(voxelotor)
300 mg
90 tablets

10PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle Label - 72786-102
NDC 72786-102-03
Oxbryta
300 mg
Swallow tablets whole.
90 tablets


