Radicava
What is Radicava (Edaravone)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study is a multicenter, randomized, double-blind, placebo-controlled trial designed to evaluate the efficacy and safety of Edaravone Dexborneol Sublingual Tablets in patients with acute ischemic stroke due to small vessel disease (TASTE-SVD). The study will enroll approximately 600 participants aged 30 to 80 years who have experienced a recent small subcortical infarct (RSSI) confirmed by MRI...
Summary: This is a multicenter, randomized, double-blind, placebo-controlled, exploratory Phase II clinical trial. The goal of this clinical trial is to assess the safety and efficacy of edaravone dexborneol sublingual tablets for post-stroke cognitive impairment in patients with acute ischemic stroke. Participants will be required to receive 24 weeks treatment of edaravone dexborneol sublingual tablets or...
Summary: EXCELLENT was a prospective, multicenter, randomized, double-blind, placebo-controlled clinical study in which participants were randomized participants were randomized (1:1) to receive either IV thrombolysis + edaravone or IV thrombolysis + matched placebo (same volume of tablets without drug components), and the primary outcome was the proportion of patients with transformed bleeding on MRI at 7...
Related Latest Advances
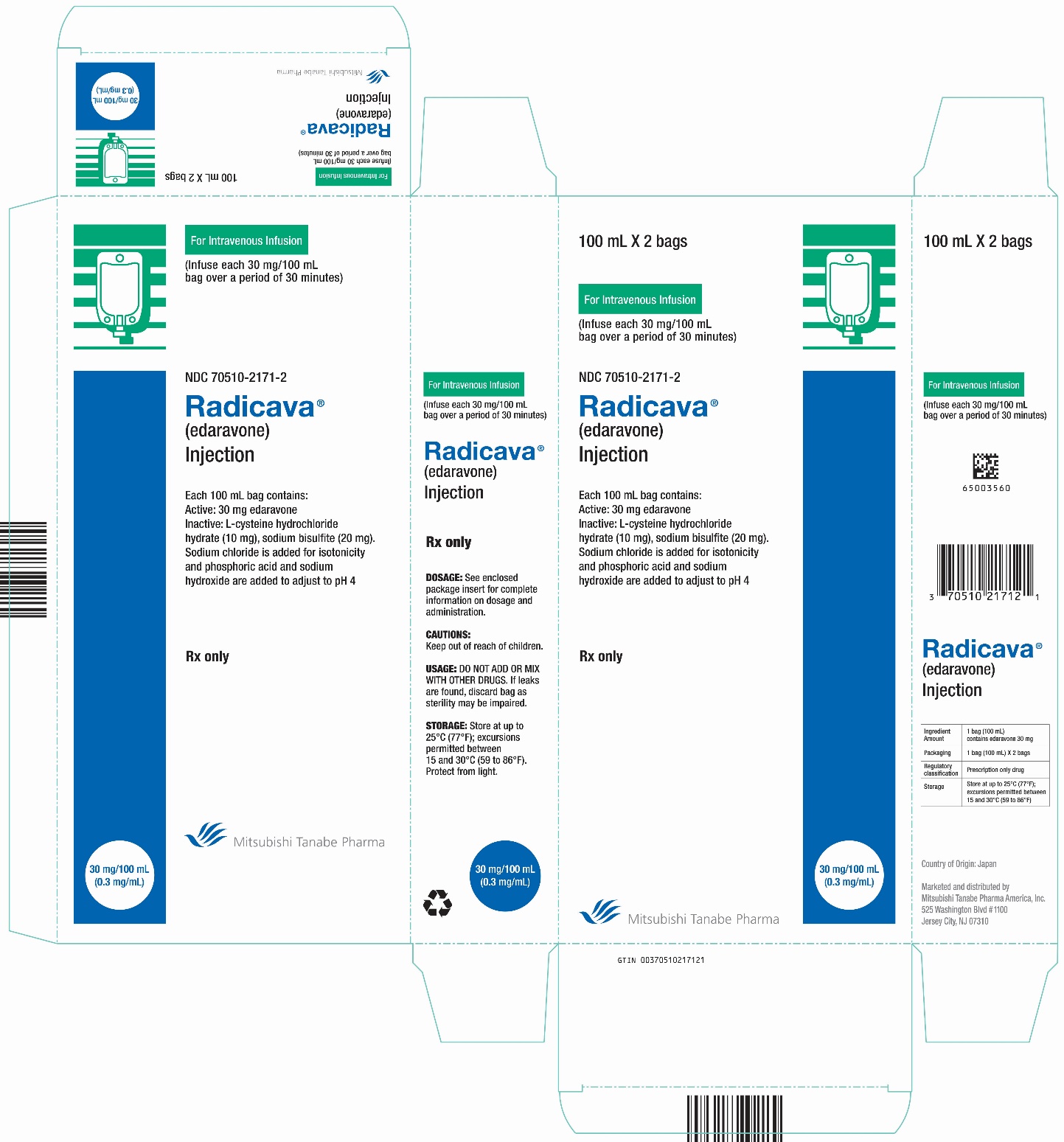
Brand Information
- Hypersensitivity Reactions
- Sulfite Allergic Reactions
- a Pooled placebo-controlled studies include two additional studies with 231 additional patients, all using the same treatment regimen [see

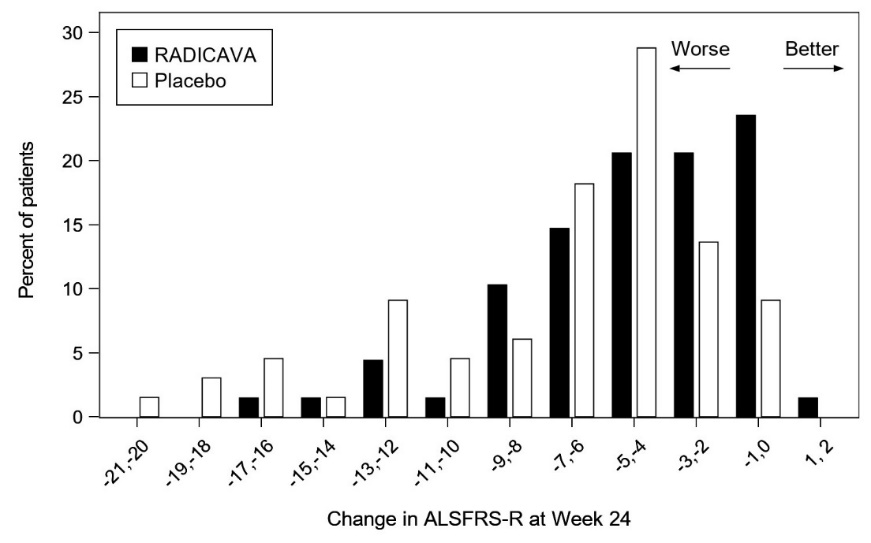
- Functionality retained most activities of daily living (defined as scores of 2 points or better on each individual item of the ALS Functional Rating Scale – Revised [ALSFRS-R; described below])
- Normal respiratory function (defined as percent-predicted forced vital capacity values of [%FVC] ≥80%)
- Definite or Probable ALS based on El Escorial revised criteria
- Disease duration of 2 years or less
- An initial treatment cycle with daily dosing for 14 days, followed by a 14-day drug-free period (Cycle 1)
- Subsequent treatment cycles with daily dosing for 10 days out of 14-day periods, followed by 14-day drug-free periods (Cycles 2-6).
- 22022-2 Iss. 11/2022
- This Patient Information has been approved by the U.S. Food and Drug Administration 22022-1 Issued: 05/2022
- Read this “Instructions for Use” before you take RADICAVA ORS for the first time and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
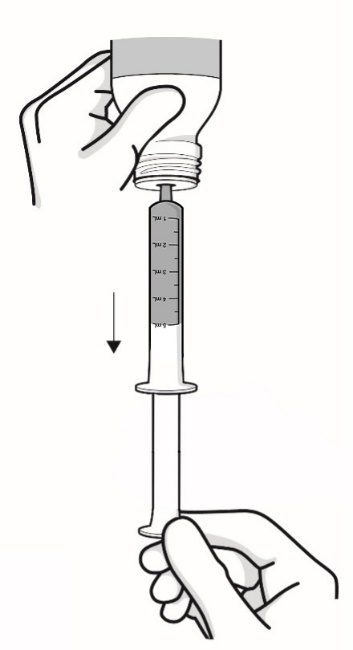
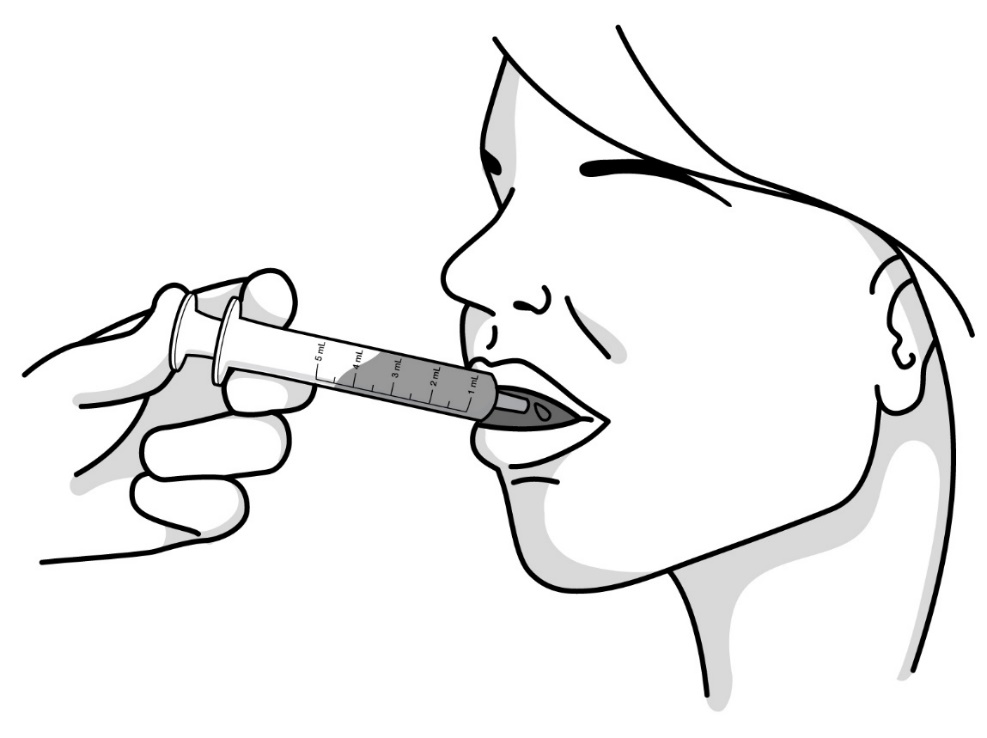
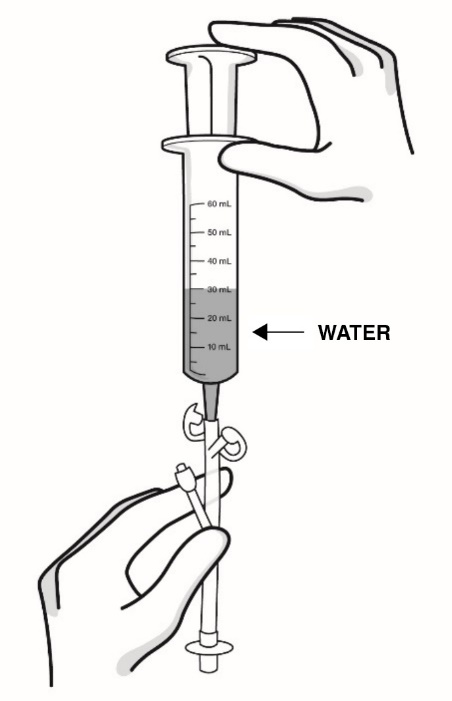
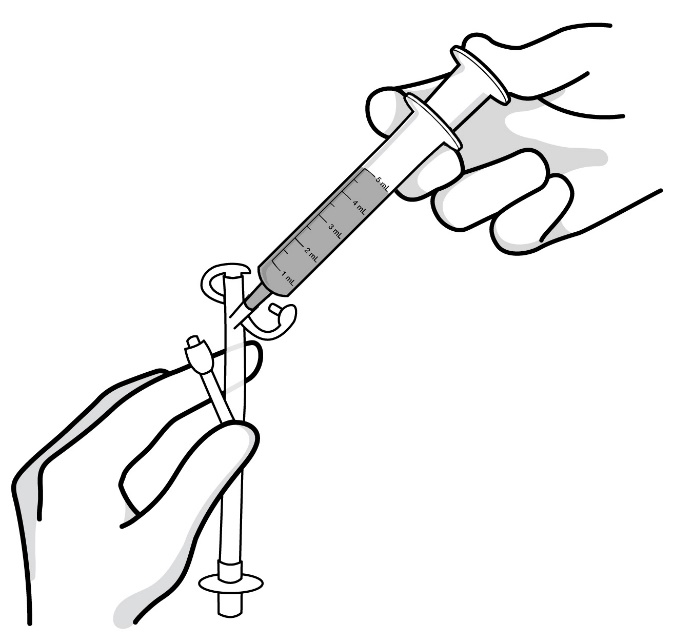
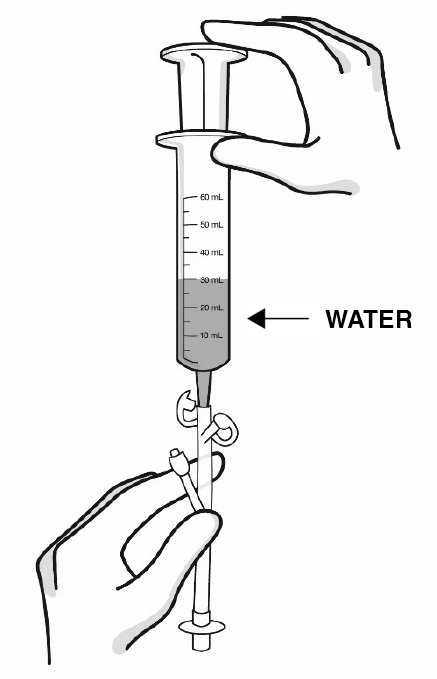
- Important information about measuring RADICAVA ORS: Always measure your prescribed dose of RADICAVA ORS using the oral syringe provided. Ask your healthcare provider or pharmacist who provided the medicine any questions you have about how to measure your prescribed dose. If you miss a dose, do not take 2 doses of RADICAVA ORS the next day. Do not take a dose of RADICAVA ORS on days 15 through 28.
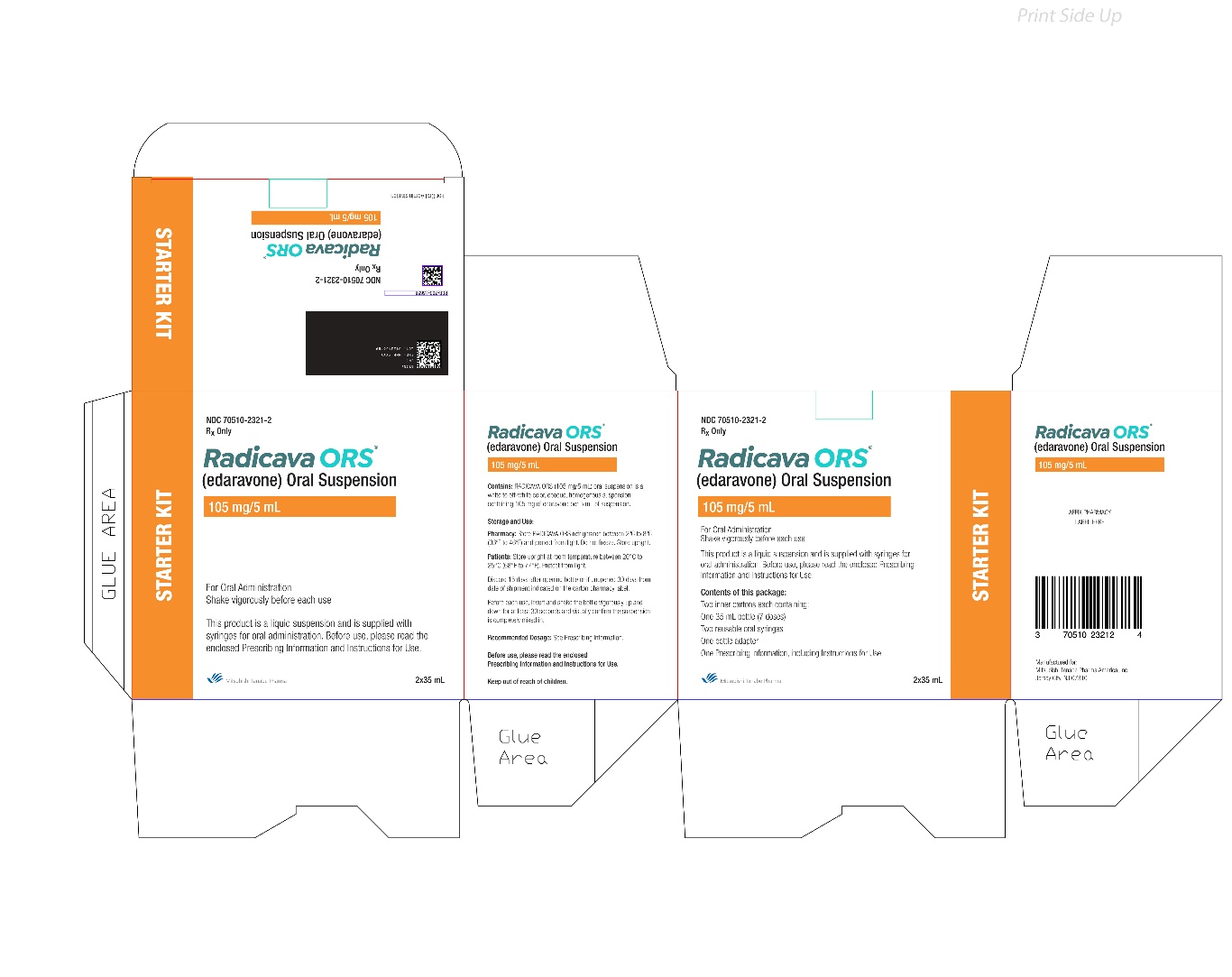
How to prepare RADICAVA ORS: Keep this Instructions for Use handy when preparing the treatment. - If your healthcare provider prescribes a Starter Kit, you will receive 2 bottles of RADICAVA ORS. Each bottle will contain 35 mL of RADICAVA ORS for a total of 70 mL to be used for your first treatment cycle of 14 days.
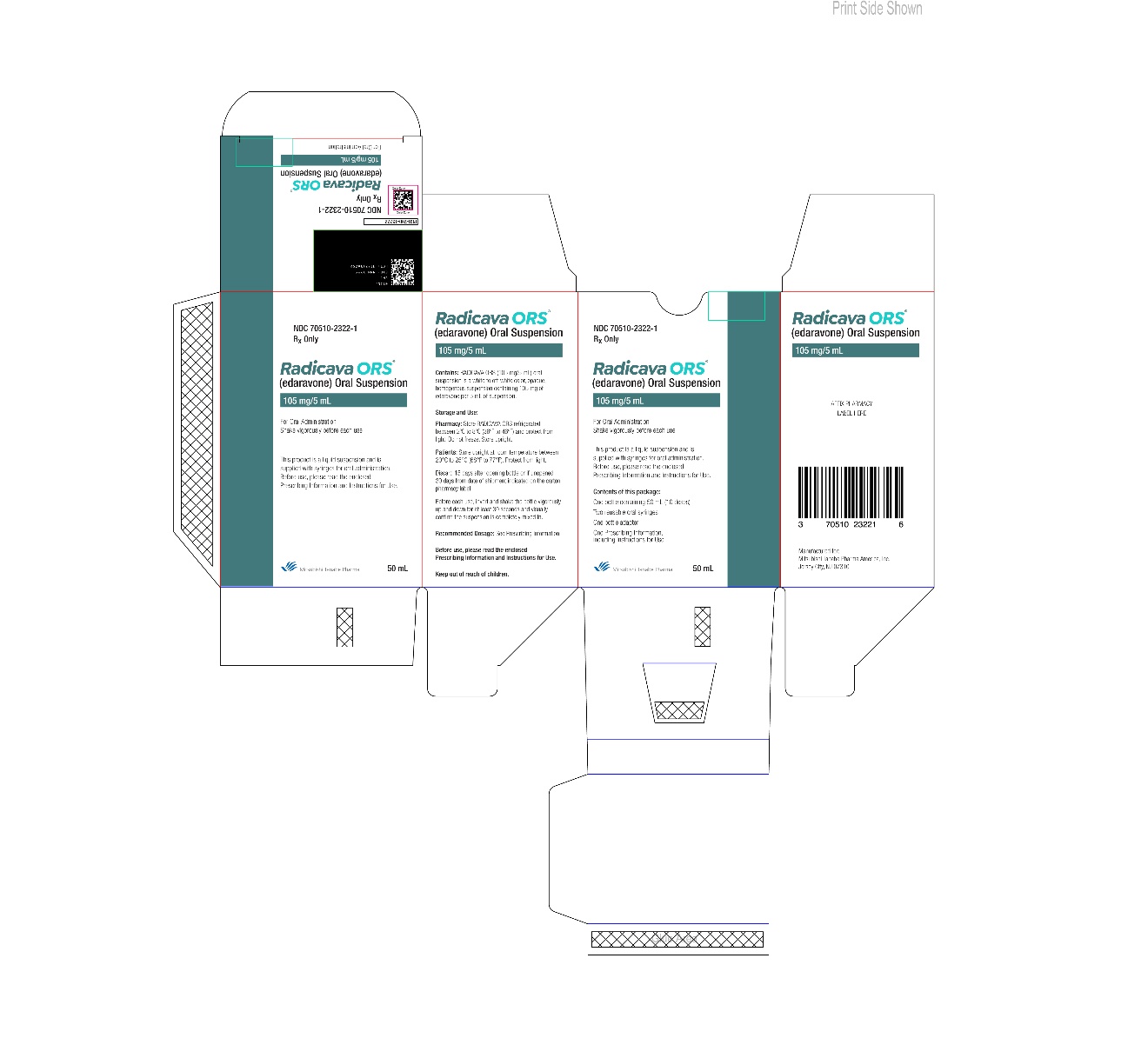
- If you were not prescribed the Starter Kit, for each treatment cycle you will receive 1 bottle of RADICAVA ORS that contains a total of 50 mL of RADICAVA ORS. After the first treatment cycle, RADICAVA ORS is to be used for 10 days out of 14 day periods.
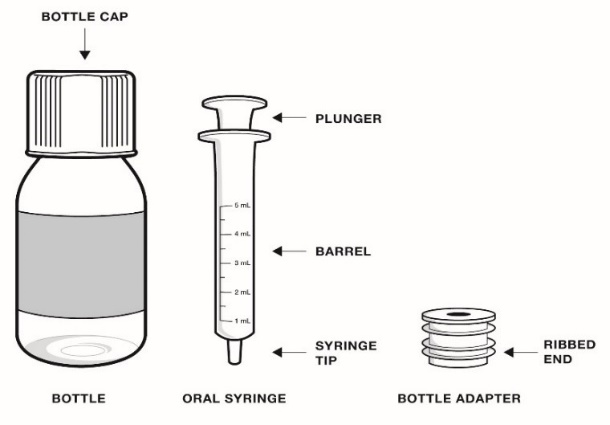
- Only use the bottle adapter and the 2 reusable 5 mL oral syringes provided with the bottle.
- Store RADICAVA ORS upright at room temperature between 68°F to 77°F (20°C to 25°C). Protect from light.
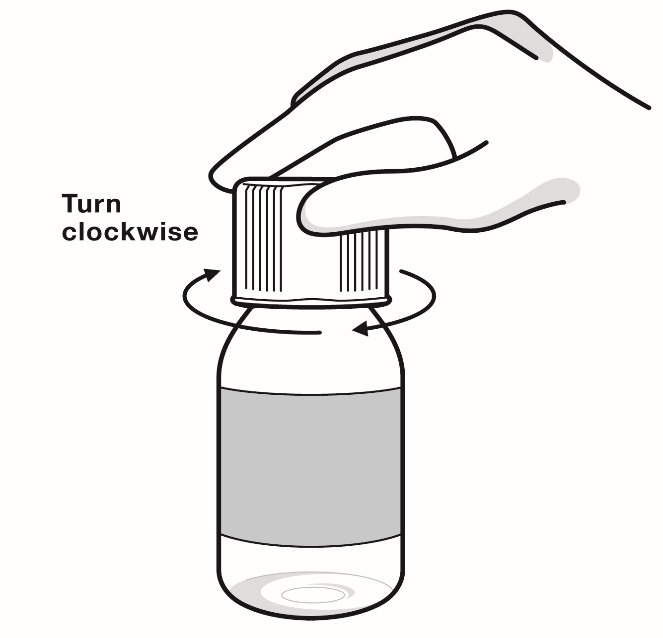
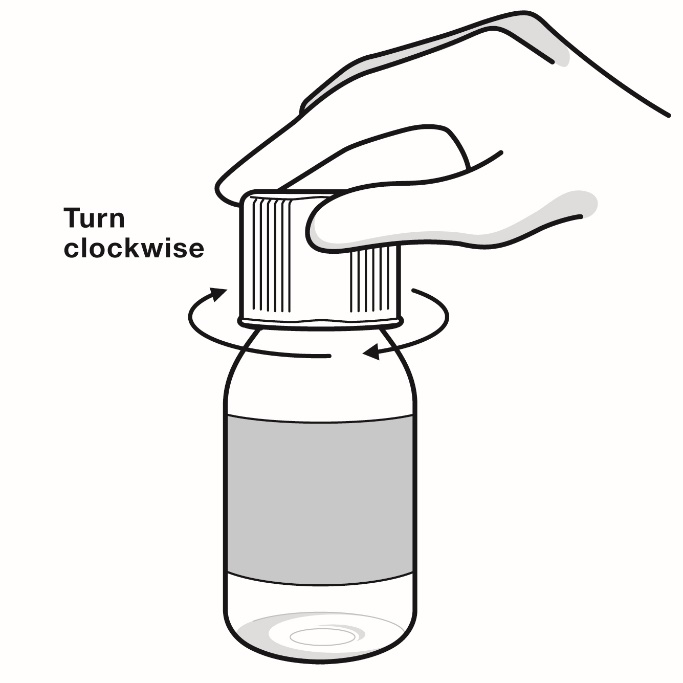
- Opening the bottle:
- When you open the bottle of RADICAVA ORS for the first use, write the date you open the bottle on the bottle label.
- After opening the bottle of RADICAVA ORS, use within 15 days.
- After a bottle of RADICAVA ORS has been opened and used, a white crust may form on the neck or on the side of the bottle. This is due to normal use and RADICAVA ORS can continue to be uses as prescribed.
- Keep bottle tightly closed between each use.
- Throw away (discard) any RADICAVA ORS that is not used within 15 days after opening the bottle or within 30 days from the date of shipment shown on the carton pharmacy label, whichever happens first. Ask your pharmacist how to properly throw away (discard) RADICAVA ORS you are no longer able to use.
- Keep RADICAVA ORS and all medicines out of the reach of children.
- 1 RADICAVA ORS bottle
- 1 bottle adapter
- 2 (5 mL) reusable oral syringes
- Keep these instructions for future use.
- Do not share RADICAVA ORS with anyone else.
- Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- People who have problems using their hands may need assistance to draw up and give the correct dose of RADICAVA ORS.
- Dosing Information:
- RADICAVA ORS has 2 different dosing schedules:
- For the first treatment cycle, you will take RADICAVA ORS every day for 14 days, followed by 14 days without the medicine.
- For the cycles after the first treatment cycle, you will take RADICAVA ORS daily for 10 out of 14 days, followed by 14 days without the medicine.
- How RADICAVA ORS will be provided:
- If your healthcare provider prescribes the Starter Kit, you will receive 2 bottles of RADICAVA ORS. Each bottle contains 35 mL of RADICAVA ORS for a total of 70 mL to deliver 14 doses.
- If you were not prescribed the starter kit, for each treatment cycle, you will receive 1 bottle of RADICAVA ORS that contains a total of 50 mL of RADICAVA ORS to deliver 10 doses.
- Fasting Information:
- Do not eat or drink anything 8 hours before each dose of RADICAVA ORS if you eat a high-fat meal.
- Do not eat or drink anything 4 hours before each dose of RADICAVA ORS if you eat a low-fat meal.
- Do not eat or drink anything 2 hours before each dose of RADICAVA ORS if you take a calorie supplement.
- You should wait at least 1 hour after taking your medicine before eating or drinking anything except water.
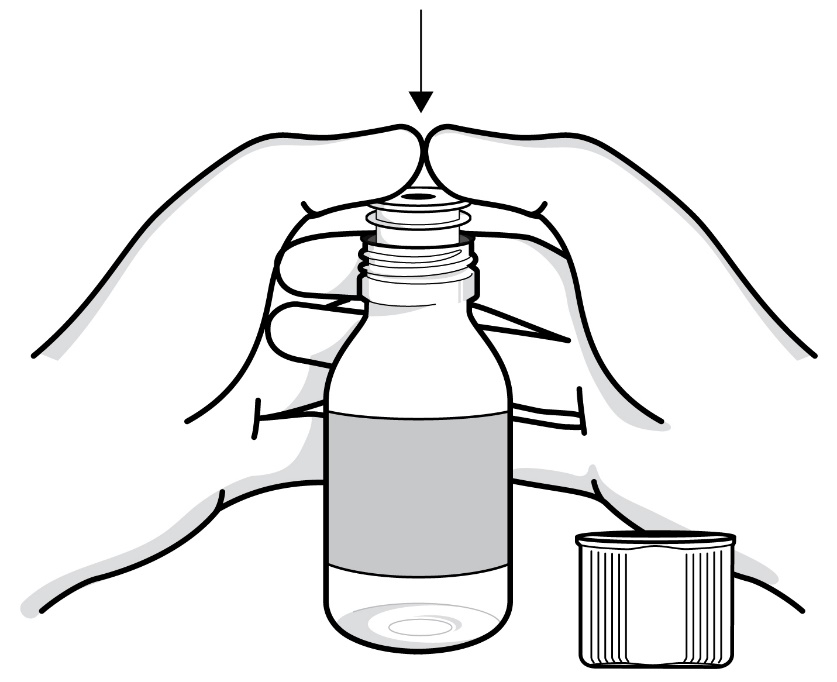

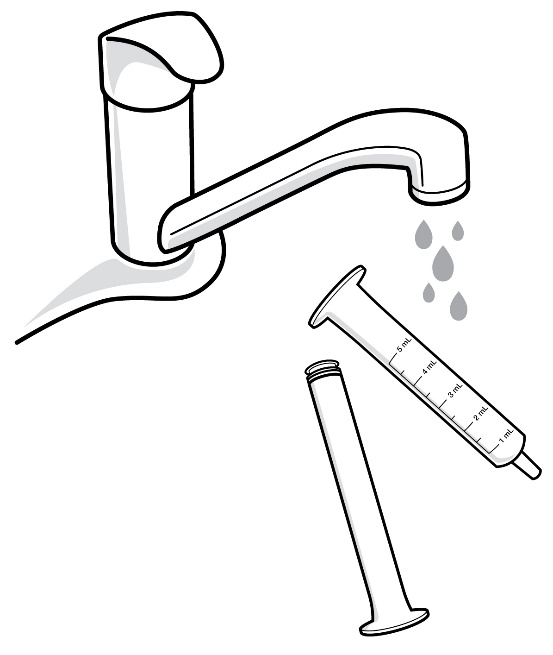
- Figure D