Brand Name
Aqneursa
Generic Name
Levacetylleucine
View Brand Information FDA approval date: September 24, 2024
Form: Granule
What is Aqneursa (Levacetylleucine)?
AQNEURSA™ is indicated for the treatment of neurological manifestations of Niemann-Pick disease type C in adults and pediatric patients weighing ≥15 kg. AQNEURSA is indicated for the treatment of neurological manifestations of Niemann-Pick disease type C in adults and pediatric patients weighing ≥15 kg.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
AQNEURSA (levacetylleucine)
1INDICATIONS AND USAGE
AQNEURSA™ is indicated for the treatment of neurological manifestations of Niemann-Pick disease type C (NPC) in adults and pediatric patients weighing ≥15 kg.
2DOSAGE FORMS AND STRENGTHS
For oral suspension: 1 gram levacetylleucine as white to off-white strawberry flavored granules in a unit-dose packet.
3CONTRAINDICATIONS
None.
4DESCRIPTION
AQNEURSA (levacetylleucine) for oral suspension contains the drug substance levacetylleucine, a modified amino acid. Levacetylleucine is slightly soluble in aqueous solutions. The chemical name is 2-acetamido-4-methylpentanoic acid. The empirical formula is C

Each packet of AQNEURSA granules contains 1 gram levacetylleucine and the inactive ingredients hypromellose, isomalt and strawberry flavor.
5CLINICAL STUDIES
The safety and efficacy of AQNEURSA for the treatment of NPC were evaluated in a randomized, double-blind, placebo-controlled, two-period crossover study (NCT05163288) that evaluated the efficacy of AQNEURSA in 60 patients. To be eligible for the study, patients had to be aged 4 years or older with a confirmed diagnosis of NPC. Patients were required to have at least mild disease-related neurological symptoms.
Patients were assessed over a 2-week baseline period. Patients were then randomized in a 1:1 ratio to one of the two treatment sequences:
- Treatment Sequence 1 (N=30): AQNEURSA in Treatment Period I, followed by immediate crossover to placebo in Treatment Period II
- Treatment Sequence 2 (N=30): placebo in Treatment Period I, followed by immediate crossover to AQNEURSA in Treatment Period II.
AQNEURSA and placebo were administered orally with or without food for 12 weeks in each period.
Patients aged ≥13 years received 4 gram per day (as 2 gram morning dose, 1 gram afternoon dose, and 1 gram evening dose). The AQNEURSA dosage in pediatric patients under 13 years was based on patient’s body weight [
Of the 60 randomized patients (37 adults and 23 pediatric patients), 27 were female and 33 were male. The median age at treatment initiation was 25 years (range: 5 to 67 years). 90% of the patients were White, 3% Asian, and 7% Other. The majority of the patients (n=51, 85%) received miglustat treatment prior to randomization and during the trial.
The primary efficacy outcome was assessed using a modified version of the Scale for Assessment and Rating of Ataxia (SARA), referred to as the functional SARA (fSARA). The SARA is a clinical assessment tool that assesses gait, stability, speech, and upper and lower limb coordination across 8 individual domains. The fSARA consists only of gait, sitting, stance, and speech disturbance domains of the original SARA with modifications to the scoring responses. Each domain was rescored from 0 to 4, where 0 is the best neurological status and 4 the worst, with a total score ranging from 0 to 16.
The fSARA score was assessed at baseline, 6 weeks, 12 weeks (the end of Period I), 18 weeks, and 24 weeks (the end of Period II). The estimated mean fSARA total score was 5.1 when patients were treated with AQNEURSA and 5.6 when patients were treated with placebo. The estimated treatment difference for the fSARA total score was -0.4 (95% CI: -0.7, -0.2) (
Patients who received AQNEURSA in Period I followed by placebo in Period II (Treatment Sequence 1) showed a greater improvement in the fSARA score in Period I with a mean change from baseline of -0.5 (SD 1.2), compared to Period II with a mean change from baseline of 0 (1.5). Similarly, patients who received placebo in Period I followed by AQNEURSA in Period II (Treatment Sequence 2) experienced greater improvement in the fSARA score while receiving AQNEURSA in Period II with a mean change of -0.7 (0.9), compared to a mean change of -0.3 (0.9) in Period I.
Figure 1 Mean (+/- standard error) plot of the fSARA total score by time and treatment sequence
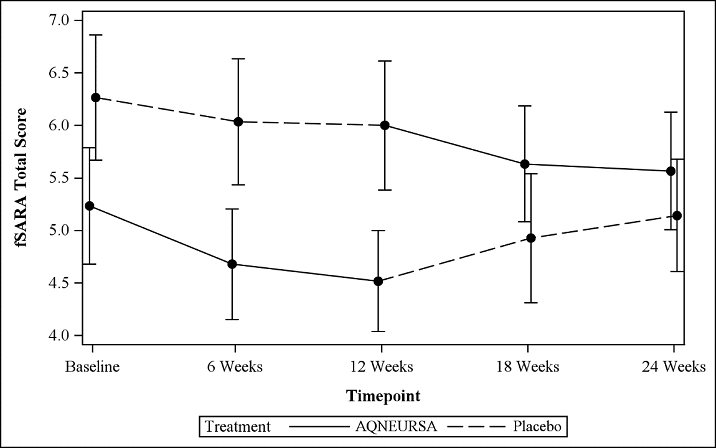
Results on the fSARA were supported by consistent results demonstrated on the original SARA.
6HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
AQNEURSA (levacetylleucine) for oral suspension is supplied as white to off-white granules in a unit-dose multi-layer aluminum/polyethylene packet. Each packet contains 1.7 gram white to off-white granules, equivalent to 1 gram levacetylleucine.
NDC 83853-101-01: Carton containing 28 unit-dose packets
Storage and Handling
Store AQNEURSA at room temperature between 20°C to 25°C (68°F to 77°F); excursion permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
7PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Instructions for Use).
Embryo-Fetal Toxicity
AQNEURSA may cause embryo-fetal harm. Advise a pregnant female of the potential risk to the fetus. Advise a female of reproductive potential and caregiver to inform their healthcare provider of a known or suspected pregnancy
Distributed by: IntraBio Inc., Austin TX 78701
AQNEURSA is a trademark of IntraBio.
8INSTRUCTIONS FOR USE
AQNEURSA [ak nur' sah]
(levacetylleucine)
for oral suspension
This Instructions for Use contains information on how to prepare and take or give AQNEURSA oral suspension. Read this Instructions for Use before you prepare and take or give the first dose of AQNEURSA oral suspension and each time you get a refill. There may be new information. This Instructions for Use does not take the place of talking to you or your child’s healthcare provider about your or your child’s medical condition or treatment.
Important Information You Need to Know Before Taking or Giving AQNEURSA Oral Suspension
- Take or give AQNEURSA oral suspension exactly as instructed by your healthcare provider.
- Take or give AQNEURSA oral suspension with or without food.
- AQNEURSA oral suspension can be taken or given by mouth or through a gastrostomy tube (G-tube) feeding tube (French size 18 or larger).
- Do not use hot liquid with AQNEURSA oral suspension.
- For instructions on disposing of expired AQNEURSA packets or unused AQNEURSA oral suspension, see the end of this Instructions for Use.
Supplies Needed to Prepare and Take or Give AQNEURSA Oral Suspension
Gather the following supplies on a clean flat surface:
- the number of AQNEURSA packets needed for your prescribed dose
- a clean spoon
- a container
- a measuring cup from your pharmacist
- 40 mL of water, orange juice, or almond milk
- catheter tip syringe (if giving AQNEURSA oral suspension through G-tube)
Storing AQNEURSA
- Store AQNEURSA at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep AQNEURSA oral suspension and all medicines out of reach of children.
Disposing of Expired AQNEURSA Packets or Unused AQNEURSA Oral Suspension
Throw away (dispose of) expired AQNEURSA packets or unused AQNEURSA oral suspension following the steps below:
- Mix medicine with a substance such as dirt, cat litter, or used coffee grounds.
- Place the mixture in a container such as a sealed plastic bag.
- Throw away (dispose of) the container in your household trash.
Distributed by: IntraBio Inc., Austin, TX 78701
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 9/2024
9PRINCIPAL DISPLAY PANEL


