Viracept
What is Viracept (Nelfinavir)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a phase I/II protocol investigating whether Nelfinavir can improve anemia and lower serum fibrosis biomarkers in Myelofibrosis patients.
Summary: This phase I trial studies the side effects and best dose of nelfinavir when given together with cisplatin and external beam radiation therapy in treating patients with vulvar cancer that has spread to nearby tissue or lymph nodes (locally advanced) and cannot be removed by surgery. Nelfinavir is an antiviral drug normally used to treat human immunodeficiency virus (HIV). Drugs used in chemotherap...
Summary: The purpose of this Phase I/II study is to determine the safety and effectiveness of up to 5 study drugs used together for the treatment of solid tumor cancers. The drugs are hydroxychloroquine, metformin, sirolimus, dasatinib and nelfinavir and are given orally.
Related Latest Advances
Brand Information
- Clinically significant adverse reactions, potentially leading to severe, life threatening, or fatal events from greater exposures of concomitant medications.
- Clinically significant adverse reactions from greater exposures of VIRACEPT.
- Loss of therapeutic effect of VIRACEPT and possible development of resistance.
- In pediatric patients ≥2 years of age receiving VIRACEPT as part of triple combination antiretroviral therapy in randomized studies, the proportion of patients achieving a HIV RNA level <400 copies/mL through 48 weeks ranged from 26% to 42%.
- Response rates in children <2 years of age appeared to be poorer than those in patients ≥2 years of age in some studies.
- Highly variable drug exposure remains a significant problem in the use of VIRACEPT in pediatric patients. Unpredictable drug exposure may be exacerbated in pediatric patients because of increased clearance compared to adults and difficulties with compliance and adequate food intake with dosing. Pharmacokinetic results from the pediatric studies are reported in Table 11
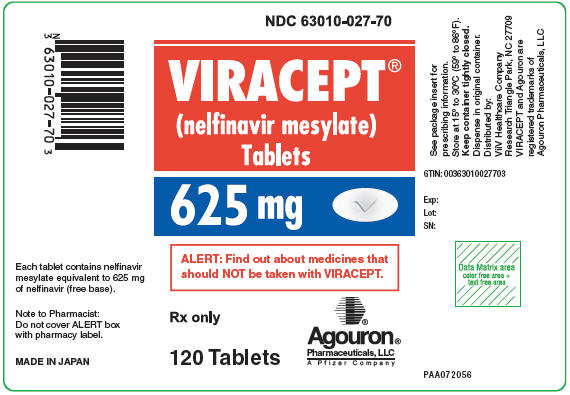
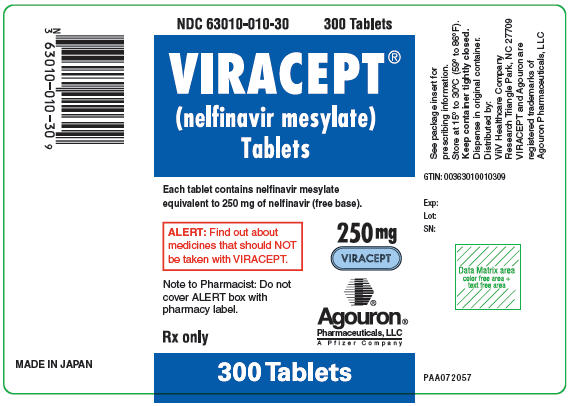
- Bottles of 300 (250 mg) tablets – NDC 63010-010-30
- Bottles of 120 (625 mg) tablets – NDC 63010-027-70
- Multiple use bottles of 144 grams of powder with scoop …….NDC 63010-011-90
- Place VIRACEPT tablet(s) in small amount of water
- Once dissolved, mix the cloudy liquid well, and consume it immediately.
- The glass should be rinsed with water and the rinse swallowed to ensure the entire dose is consumed
- Mix VIRACEPT Oral Powder with a small amount of water, milk, formula, soy formula, soy milk, or dietary supplements
- Once mixed, the entire contents must be consumed in order to obtain the full dose.
- If the mixture is not consumed immediately, it must be stored under refrigeration, but storage must not exceed 6 hours.
- Acidic food or juice (e.g., orange juice, apple juice, or apple sauce) are not recommended for mixing VIRACEPT Oral Powder because the combination may result in a bitter taste.
- VIRACEPT Oral Powder should not be reconstituted with water in its original container.
Pfizer
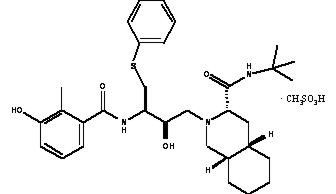
(nelfinavir mesylate)
Tablets
should NOT be taken with VIRACEPT.
(nelfinavir mesylate)
Tablets
should NOT be taken with VIRACEPT.