Brand Name
Miplyffa
Generic Name
Arimoclomol
View Brand Information FDA approval date: September 20, 2024
Form: Capsule
What is Miplyffa (Arimoclomol)?
MIPLYFFA is indicated for use in combination with miglustat for the treatment of neurological manifestations of Niemann-Pick disease type C in adult and pediatric patients 2 years of age and older. MIPLYFFA is indicated for use in combination with miglustat for the treatment of neurological manifestations of Niemann-Pick disease type C in adult and pediatric patients 2 years of age and older.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
MIPLYFFA (arimoclomol citrate)
1INDICATIONS AND USAGE
MIPLYFFA is indicated for use in combination with miglustat for the treatment of neurological manifestations of Niemann-Pick disease type C (NPC) in adult and pediatric patients 2 years of age and older.
2DOSAGE FORMS AND STRENGTHS
MIPLYFFA (arimoclomol) capsules are available as follows:
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described below and elsewhere in the labeling:
- Hypersensitivity Reactions
- Increased Creatinine without Affecting Glomerular Function
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of MIPLYFFA was evaluated in a randomized, double-blind, placebo-controlled, 12-month trial (Trial 1), which included 50 patients 2 to 19 years old with NPC
The most common adverse reactions in Trial 1 (≥ 15%) in MIPLYFFA-treated patients who also received miglustat were upper respiratory tract infection, diarrhea, and decreased weight. Three (6%) of the MIPLYFFA-treated patients had the following adverse reactions that led to withdrawal from Trial 1: increased serum creatinine (one patient), and progressive urticaria and angioedema (two patients). Serious adverse reactions reported in MIPLYFFA-treated patients in Trial 1 were hypersensitivity reactions including urticaria and angioedema.
Table 1shows common adverse reactions in Trial 1 that occurred in at least 8% of MIPLYFFA-treated patients who also received miglustat.
5DESCRIPTION
MIPLYFFA capsules contain arimoclomol citrate. Arimoclomol citrate is a crystalline powder of white to off-white color that is freely soluble in water. The chemical name is
The empirical formula is C
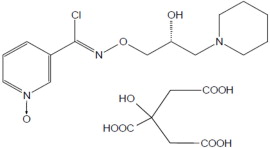
MYPLIFFA contains 47 mg, 62 mg, 93 mg, or 124 mg of arimoclomol (equivalent to 75 mg, 100 mg, 150 mg, or 200 mg of arimoclomol citrate). The inactive ingredients are microcrystalline cellulose (MCC) and magnesium stearate. The inactive ingredients are not water soluble and will remain undissolved if the contents of the capsule are added to beverages or soft food
The capsule shells contain hypromellose, titanium dioxide, and one or more of the following: Brilliant Blue FCF-FD&C Blue 1, Yellow iron oxide and Red iron oxide.
6CLINICAL STUDIES
Safety and effectiveness of MIPLYFFA were assessed in Trial 1, a randomized, double-blind, placebocontrolled, 12-month trial in patients 2 to 19 years of age who had a molecularly confirmed diagnosis of NPC (NCT02612129). Fifty patients were randomized 2:1 to treatment with weight-adjusted MIPLYFFA (31 to 124 mg) or placebo orally three times per day. The randomization was stratified by miglustat use status at baseline.
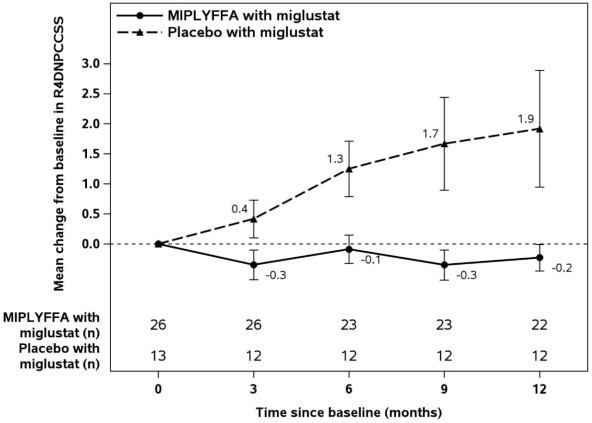
Efficacy assessments, including the rescored 4-domain NPC Clinical Severity Scale (R4DNPCCSS) score, were performed at baseline and every 3 months until 12 months of treatment. The R4DNPCCSS is a measure of NPC disease progression that consists of the four items assessing ambulation, speech, swallow, and fine motor skills that patients with NPC and their caregivers and physicians have identified as most relevant with higher scores representing greater severity of disease.
In Trial 1, 76% and 81% of patients in the MIPLYFFA and placebo groups, respectively, received miglustat six months or longer prior to the time of enrollment. For the subgroup of patients who also received miglustat at enrollment, the mean age was 11.6 years, the mean time since first NPC symptom was 8.5 years, and the mean age at onset of first neurological symptom was 4.9 years. In this subgroup, 56% of patients were females, 87% were white, 5% were Asian, 3% were native Hawaiian or other Pacific Islander, and 5% were unknown. The mean baseline R4DNPCCSS score was higher in the MIPLYFFA group (n=26; mean=8.9) than the placebo group (n=13; mean=7), with an overall mean R4DNPCCSS score of 8.3.
In the MIPLYFFA group, four patients discontinued the study: one patient due to consent withdrawal and three patients due to adverse reactions
Table 3displays the change from baseline in R4DNPCCSS score at month 12 in patients 2 to
19 years of age with NPC who also received miglustat in Trial 1. See
Figure 1: MIPLYFFA and Placebo Mean Change from Baseline (± SE) in R4DNPCCSS Score Over Time in Patients 2 to 19 Years of Age with NPC Who Also Received Miglustat (Trial 1)
There were insufficient data to determine the effectiveness of the use of MIPLYFFA without miglustat for the treatment of neurological manifestations in patients with NPC.
7PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (